Abstract
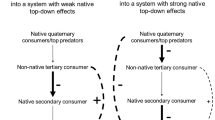
Non-native species are recognized as important components of change to food web structure. Non-native prey may increase native predator populations by providing an additional food source and simultaneously decrease native prey populations by outcompeting them for a limited resource. This pattern of apparent competition may be important for plants and sessile marine invertebrate suspension feeders as they often compete for space and their immobile state make them readily accessible to predators. Reported studies on apparent competition have rarely been examined in biological invasions and no study has linked seasonal patterns of native and non-native prey abundance to increasing native predator populations. Here, we evaluate the effects of non-native colonial ascidians (Diplosoma listerianum and Didemnum vexillum) on population growth of a native predator (bloodstar, Henricia sanguinolenta) and native sponges through long-term surveys of abundance, prey choice and growth experiments. We show non-native species facilitate native predator population growth by providing a novel temporal resource that prevents loss of predator biomass when its native prey species are rare. We expect that by incorporating native and non-native prey seasonal abundance patterns, ecologists will gain a more comprehensive understanding of the mechanisms underlying the effects of non-native prey species on native predator and prey population dynamics.








Similar content being viewed by others
References
Anderson JM (1960) Histological studies on the digestive system of a starfish, Henricia, with notes on Tiedemann’s pouches in starfishes. Biol Bull 119:371–398
Baiser B, Russell GJ, Lockwood JL (2010) Connectance determines invasion success via trophic interactions in model food webs. Oikos 119:1970–1976
Bak R, Lambrechts D, Joenje M, Nieuwland G, Van Veghel M (1996) Long-term changes on coral reefs in booming populations of a competitive colonial ascidian. Mar Ecol Prog Ser 133:303–306
Dangremond EM, Pardini EA, Knight TM (2010) Apparent competition with an invasive plant hastens extinction of an endangered lupine. Ecology 91:2261–2271
Dijkstra JA, Harris LG (2009) Maintenance of diversity altered by a shift in dominant species: implications for species coexistence. Mar Ecol Prog Ser 387:71–80
Dijkstra J, Harris LG, Westerman E (2007) The distribution and long-term temporal patterns of four invasive colonial ascidians in the Gulf of Maine. J Exp Mar Biol Ecol 342:61–68
Dijkstra JA, Westerman EL, Harris LG (2011) The effects of climate change on species composition, succession and phenology: a case study. Glob Change Biol 17:2360–2369
Fogarty M, Incze L, Hayhoe K, Mountain D, Manning J (2008) Potential climate change impacts on Atlantic cod (Gadus morhua) off the northeastern USA. Mitig Adapt Strat Glob Change 13:453–466
Grosholz ED (2002) Ecological consequences of coastal invasions. Trends Ecol Evol 17:22–27
Hamilton J, Halzapfel C, Mahall BE (1999) Coexistence and interference between a native perennial grass and non-native annual grasses in California. Oecologia 121:518–526
Harris LG, Tyrrell MC (2001) Changing community states in the gulf of maine: synergisms between invaders, overfishing and climate change. Biol Invasions 3:9–21
Holt RD, Kotler BP (1987) Short-term apparent competition. Am Nat 130:412–430
Hughes RN (1979) Optimal diets under the energy maximization premise: the effects of recognition time and learning. Am Nat 113:209–221
Hurlbert AW (1980) The functional role of asterias vulgaris (Verrill 1866) in three subtidal communities. Dissertation, University of New Hampshire, Durham
Inger R, McDonald R, Rogowski D, Jackson A, Parnell A, Preston S, Harrod C, Goodwin C, Griffiths D, Dick J, Elwood R, Newton J, Bearhop S (2010) Do non-native invasive fish support elevated lamprey populations? J Appl Ecol 47:121–129
Lambert WJ, Todd CD, Thorpe JP (2000) Variation in growth rate and reproductive output in British populations of the dorid nudibranch Adalaria proxima: consequences of restricted larval dispersal? Mar Biol 137:149–159
Lechowicz MJ, Koike T (1995) Phenology and seaonality of woody plants: an unappreciated element in global change research? Can J Bot 73:147–148
Mah C, Hansson H (2011) Henricia sanguinolenta (O.F. Müller, 1776). In: Mah CL (ed) World register of marine species. Available at http://www.marinespecies.org/aphia.php?p=taxdetails&id=123974
Moore PJ, Thompson RC, Hawkins SJ (2011) Phenological changes in intertidal con-specific gastropods in response to climate warming. Glob Change Biol 17:709–717
Pershing AJ, Greene CH, Jossi JW, O’Brien L, Broziak JKT, Bailey BA (2005) Interdecadal variability in the Gulf of Maine zooplankton community, with potential impacts on fish recruitment. J Mar Sci 62:1511–1523
Rilov G (2009) Predator-prey interactions in marine bioinvasions. In: Rilov G, Crooks JA (eds) Biological invasions in marine ecosystems: ecological management and geographic perspectives. Springer, Heidelberg, pp 261–285
Roemer GW, Donlan CJ, Courchamp F (2002) Golden eagles, feral pigs, and insular carnivores: how exotic species turn native predators into prey. Proc Nat Acad Sci 99:791–796
Ruiz GM, Carlton JT, Grosholz ED, Hines AH (1997) Global invasions of marine and estuarine habitats by non-indigenous species: mechanisms, extent, and consequences. Am Zool 37:621–632
Shield C, Witman J (1993) The impact of Henricia sanguinolenta (O.F. Muller) (Echinodermata: Asteroidea) predation on the finger sponges, Isodictya spp. J Exp Mar Biol Ecol 66:107–133
Shurin JB, Borer ET, Seabloom EW (2002) A cross ecosystem comparison of the strength of trophic cascades. Ecol Lett 5:785–791
Sorte CJB, Williams SL, Zerebecki RA (2010) Ocean warming increases threat of invasive species in a marine fouling community. Ecology 91:2198–2204
Stachowicz JJ, Byrnes JE (2006) Species diversity, invasion success, and ecosystem functioning: disentangling the influence of resource competition, facilitation, and extrinsic factors. Mar Ecol Prog Ser 311:251–262
Tonn WM, Magnuson JJ (1982) Patterns in the species composition and richness of fish assemblages in northern Wisconsin lakes. Ecology 63:1149–1166
Vasserot J (1961) Caractere hautement specialise du regime alimentaire chez les asterides Echinaster sepositus et Henricia sanguinolenta, predateurs de spongiares. Bulletin de la Societe Zoologique de France 86:796–809
Westerman EL, Whitlatch RB, Dijkstra JA, Harris LG (2009) Variation in brooding period masks similarities in response to changing temperatures. Mar Ecol Prog Ser 391:13–19
Wethey DS, Walters LJ (1986) Quantifying spatial patterns of overgrowth in epibenthic communities. Mar Ecol Prog Ser 29:271–278
Witman J, Sebens K (1990) Distribution and ecology of sponges at a subtidal rock in central Gulf of Maine. In: Rutzler K (ed) New perspectives in sponge biology. Smithsonian Institution, Washington
Acknowledgments
We would like to thank the Shoals Marine Laboratory and the class of Field Marine Biology and Ecology (instructors: C. Siddon and K. A. Miller) for collecting the intertidal data from the Isles of Shoals. The Shoals Marine Laboratory provided J.A. Dijkstra with housing and space. We thank H. Weeks for his critical comments on earlier drafts of the manuscript. We would also like to thank Dr. Jim Carlton and an anonymous reviewer for their helpful and constructive criticism. Finally, we would like to thank our dive partners: J. Friedman, C. Brooks, J. Mercer, O. Rhoades and C. Keough and O. Lambert for lab assistance. We thank S. J. Dijkstra and E. L.Westerman for their comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dijkstra, J.A., Lambert, W.J. & Harris, L.G. Introduced species provide a novel temporal resource that facilitates native predator population growth. Biol Invasions 15, 911–919 (2013). https://doi.org/10.1007/s10530-012-0339-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-012-0339-1