Abstract
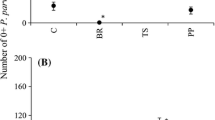
Understanding the mechanisms behind the successful colonization and establishment of introduced species is important for both preventing the invasion of unwanted species and improving release programs for biological control agents. However, it is often not possible to determine important introduction details, such as date, number of organisms, and introduction location when examining factors affecting invasion success. Here we use biological control introduction data to assess the role of propagule pressure, disturbance, and residence time on invasion success of four herbivorous insect species introduced for the control of the invasive wetland plant, Lythrum salicaria, in the Columbia River Estuary. Two sets of field surveys determined persistence at prior release sites, colonization of new sites, and abundance within colonized sites. We quantified propagule pressure in four ways to examine the effect of different measurements. These included three measurements of introduction size (proximity to introduction site, introduction size at a local scale, and introduction size at a regional scale) and one measure of introduction number (number of introduction events in a region). Disturbance was examined along a tidal inundation gradient (distance from river mouth) and as habitat (island or mainland). Statistical models and model averaging were used to determine which factors were driving invasion success. In this study we found: (1) sparse evidence for the positive influence of propagule pressure on invasion success; (2) disturbance can negatively affect the invasion success of herbivorous insects; (3) the effects of disturbance and propagule pressure are species specific and vary among invasion stages, and (4) not all measures of propagule pressure show the same results, therefore single measures and proxies should be used cautiously.





Similar content being viewed by others
References
Albright MF, Harman WN, Fickbohm SS, Meehan H, Groff S, Austin T (2004) Recovery of native flora and behavioral responses by Galerucella spp. following biological control of purple loosestrife. Am Midl Nat 152(2):248–254
Beirne BP (1975) Biological control attempts by introductions against pest insects in the field in Canada. Can Entomol 107:225–236
Blossey B (1995) Coexistence of two leaf-beetles in the same fundamental niche, distribution, adult phenology, and oviposition. Oikos 74:225–234
Blossey B, Skinner LC, Taylor J (2001) Impact and management of purple loosestrife (Lythrum salicaria) in North America. Biodivers Conserv 10:1787–1807
Britton-Simmons KH, Abbott KC (2008) Short- and long-term effects of disturbance and propagule pressure on a biological invasion. J Ecol 96:68–77
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information theoretic approach, 2nd edn. Springer, New York
Chawala A, Jay AD, Baptista AM, Wilkin M, Seaton C (2008) Seasonal variability and estuary-shelf interactions in circulation dynamics of a River-dominated estuary. Estuar Coasts 31:269–288
Chytrý M, Jarošík V, Pyšek P, Hájek O, Knollová I, Tichý L, Danihelka J (2008) Separating habitat invisibility by alien plants from the actual level of invasion. Ecology 89(6):1541–1553
Crawley MJ (2005) Statistics: an introduction using R. Wiley, West Sussex
Crawley MJ, Kornberg H, Lawton JH, Usher MB, Southwood R, O’Connor RJ, Gibbs A (1986) The population biology of invaders (and discussion). Philos Trans R Soc Lond Ser B Biol Sci 314(1167):711–731
Dawson W, Burslem DFRP, Hulme PE (2009) Factors explaining alien plant invasion success in a tropical ecosystem differ at each stage of invasion. J Ecol 97:657–665
De Clerck-Floate R, Wikeem B (2009) Influence of release size on establishment and impact of a root weevil for the biological control of houndstongue (Cynoglossum officinale). Biol Sci Technol 19(2):169–183
Dehnen-Schmutz K, Touza J, Perrings C, Williams M (2007) A century of the ornamental plant trade and its impact on invasion success. Divers Distrib 13:527–534
Denoth M, Myers JH (2005) Variable success of biological control of Lythrum salicaria in British Columbia. Biol Control 32:269–279
Edward E, Munishi KT, Hulme PE (2009) Relative roles of disturbance and propagule pressure on the invasion of humid tropical forest by Cordia alliodora (Boraginaceae) in Tanzania. Biotropica 41(2):171–178
Fagan WF, Lewis MA, Neubert MG, van den Driessche P (2002) Invasion theory and biological control. Ecol Lett 5:148–157
Ferrarese E, Garono RJ (2010) Dispersal of Galerucella pusilla and G. calmariensis via passive water transport in the Columbia River Estuary. Biol Control 52(2):115–122
Gelman A, Hill J (2007) Data analysis using regression and multilevel/hierarchical models. Cambridge University Press, New York
Grevstad FS (1999a) Experimental invasions using biological control introductions: the influence of release size on the chance of population establishment. Biol Invasions 1:313–323
Grevstad FS (1999b) Factors influencing the chance of population establishment: implications for release strategies in biological control. Ecol Appl 9(4):1439–1447
Grevstad FS, Herzig AL (1997) Quantifying the effects of distance and conspecific on colonization: experiments and models using the loosestrife leaf beetle, Galerucella calmariensis. Oecologia 110:60–68
Groves RH (2006) Are some weeds sleeping? Some concepts and reasons. Euphytica 148:111–120
Hayes KR, Barry SC (2008) Are there any consistent predictors of invasion success? Biol Invasions 10:483–506
Hylander K (2009) No increase in colonization rate of boreal bryophytes close to propagule sources. Ecology 90:160–169
Jeschke JM, Strayer DL (2006) Determinants of vertebrate invasion success in Europe and North America. Global Change Biol 12:1608–1619
Kolar CS, Lodge DM (2001) Progress in invasion biology: predicting invaders. Trends Ecol Evol 16(4):199–204
Lande R (1993) Risks of population extinction from demographic and environmental stochasticity and random catastrophes. Am Nat 142(6):911–927
Leung B, Drake JM, Lodge DM (2004) Predicting invasions: propagule pressure and the gravity of Alee effects. Ecology 85(6):1651–1660
Liebhold AM, Tobin PC (2008) Population ecology of insect invasions and their management. Annu Rev Entomol 53:387–408
Lockwood JL, Cassey P, Blackburn T (2005) The role of propagule pressure in explaining species invasions. Trends Ecol Evol 20(5):223–228
Lozon JD, Maclsaac HJ (1997) Biological invasions: are they dependent on disturbance? Environ Rev 5:131–144
Mack RN, Simberloff D, Londsdale WM, Evans H, Clout M, Bazzaz FA (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10(3):689–710
Malecki RA, Blossey B, Hight SD, Schroeder D, Kok LT, Coulson JR (1993) Biological control of purple loosestrife. Bioscience 43:680–686
Martin TG, Wintle BA, Rhodea JR, Kuhnert PM, Field SA, Low-Choy SJ, Tyre AJ, Possingham HP (2005) Zero tolerance ecology: improving ecological inference by modeling the source of zero observations. Ecol Lett 8:1235–1246
Melbourne BA, Cornell HV, Davies KF, Dugaw CJ, Elmendorf S, Freestone AL, Hall R, Harrison S, Hastings A, Holland M, Holyoak M, Lambrinos J, Moore K, Yokomizo H (2007) Invasion in a heterogeneous world: resistance, coexistence or hostile takeover? Ecol Lett 10(1):77–94
Memmott J, Fowler SV, Hill RL (1998) The effect of release size on the probability of establishment of biological control agents: gorse thrips (Sericothrips staphylinus) released against gorse (Ulex europaeus) in New Zealand. Biocontrol Sci Tech 8:103–115
Mikheyev AS, Tchingnoumba L, Henderson A, Alonso A (2008) Effect of propagule pressure on the establishment and spread of the little fire ant Wasmannia auropunctata in a Gabonese oilfield. Divers Distrib 14:301–306
Milton SJ, Wilson JRU, Richardson DM, Seymour CL, Dean WR, Iponga DM, Procheş Ş (2007) Invasive alien plants infiltrate bird-mediated shrub nucleation processes in arid savanna. J Ecol 95:648–661
Morin L, Reid AM, Sims NM, Buckley YM, Dhileepan K, Hastwell GT, Nordblom TL, Raghu S (2009) Review of approaches to evaluate the effectiveness of weed biological control agents. Biol Control 51(1):1–15
Myburgh M, Chown SL, Daniels SR, Jansen van Vuuren B (2007) Population structure, propagule pressure, and conservation biogeography in the sub-Antarctic: lessons from indigenous and invasive springtails. Divers Distrib 13:143–154
Pemberton RW, Liu H (2009) Marketing time predicts naturalization of horticultural plants. Ecology 90:69–80
Piper GL, Coombs EM, Blossey B, McEvoy PB, Schooler SS (2004) Purple loosestrife. In: Coombs EM, Clark JK, Piper GL, Cofrancesco AF (eds) Biological control of invasive plants in the United States. Oregon State University Press, Corvallis, pp 281–292
Pyšek P, Jarošík V (2005) Residence time determines the distribution of alien plants. In: Inderjit S (ed) Invasive plants: ecological and agricultural aspects. Birkhäuser Basel, Switzerland, pp 77–96
Pyšek P, Jarošík V, Müllerová J, Pergl J, Wild J (2008) Comparing the rate of invasion by Heracleum mantegazziam at continental, regional, and local scales. Divers Distrib 14:355–363
R Development Core Team (2008) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, http://www.R-project.org
Rouget M, Richardson DM (2003) Inferring process from pattern in plant invasions: a semimechanistic model incorporating propagule pressure and environmental factors. Am Nat 162:713–724
Ruesink JL (2005) Global analysis of factors affecting the outcome of freshwater fish introductions. Conserv Biol 19(6):1883–1893
Sax DF, Gaines SD (2008) Species invasions and extinction: the future of native biodiversity on islands. Proc Natl Acad Sci USA 105:11490–11497
Schooler SS, McEvoy PB, Coombs EM (2006) Negative per capita impacts of purple loosestrife and reed canary grass on plant diversity of wetland communities. Divers Distrib 12:351–363
Schooler SS, McEvoy PB, Hammond P, Coombs EM (2009) Negative per capita effects of two invasive plants, Lythrum salicaria and Phalaris arundinacea, on the moth diversity of wetland communities. Bull Entomol Res 99:229–243
Shea K, Possingham HP (2000) Optimal release strategies for biological control agents: an application of stochastic dynamic programming to population management. J Appl Ecol 37:77–86
Stokes KE, Cunningham SA (2006) Predictors of recruitment for willows invading riparian environments in south-east Australia: implications for weed management. J Appl Ecol 43:909–921
Turner MG, Gardner RH, O’Neill RV (2001) Landscape ecology in theory and practice: pattern and process. Springer-Verlag, New York
Whittingham MJ, Swetnam RD, Wilson JD, Chamberlain DE, Freckleton RP (2005) Habitat selection by yellowhammers Emberiza citronella on lowland farmland at two spatial scales: implications for conservation management. J Appl Ecol 42(2):270–280
Williamson M, Fitter A (1996) The varying success of invaders. Ecology 77(6):1661–1666
Wilson JRU, Richardson DM, Rouget M, Procheş Ş, Amis MA, Henderson L, Thuiller W (2007) Residence time and potential range: crucial considerations in modelling plant invasions. Divers Distrib 13:11–22
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgments
We thank J. Kooser, L. Moore, and E. Thompson for laboratory and field assistance. G. Dorsey, E. Coombs, P. McEvoy, F. Grevstad, L. Wunder, D. McLain, and M. Magruder provided valuable support and advice. Special thanks to A. Bourne, S. Burgess, and J. Dwyer for statistical advice. R. Van Klinken and three anonymous reviewers provided constructive reviews. Funding for this project was provided to S. Schooler and R. Garono by the Oregon Department of Agriculture and the Oregon State Weed Board, to A. Yeates through an Australian Postgraduate Award, and to Y. Buckley by an ARC Australian Research Fellowship (DP0771387).
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
See Table 3.
Rights and permissions
About this article
Cite this article
Yeates, A.G., Schooler, S.S., Garono, R.J. et al. Biological control as an invasion process: disturbance and propagule pressure affect the invasion success of Lythrum salicaria biological control agents. Biol Invasions 14, 255–271 (2012). https://doi.org/10.1007/s10530-011-0060-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-011-0060-5