Abstract
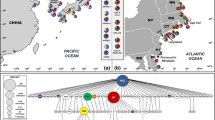
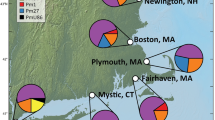
Surveys of genetic structure of introduced populations of nonindigenous species may reveal the source(s) of introduction, the number of introduction events, and total inoculum size. Here we use the mitochondrial cytochrome c oxidase subunit 1 (COI) gene to explore genetic structure and contrast invasion histories of two ecologically similar and highly invasive colonial ascidians, the golden star tunicate Botryllus schlosseri and the violet tunicate Botrylloides violaceus, in their global and introduced North American ranges. Haplotype and nucleotide diversities for B. schlosseri were significantly higher than for B. violaceus both globally (h = 0.872; π = 0.054 and h = 0.461; π = 0.007, respectively) and in their overlapping North American ranges (h = 0.874; π = 0.012 and h = 0.384; π = 0.006, respectively). Comparative population genetics and phylogenetic analyses revealed clear differences in patterns of invasion for these two species. B. schlosseri populations on the west and east coasts of North America were seeded from the Pacific and Mediterranean regions, respectively, whereas all North American B. violaceus populations were founded by one or more introduction events from Japan. Differences in genetic structure of invasive populations for these species in North America are consistent with their contrasting probable introduction vectors. B. schlosseri invasions most likely resulted from vessel hull fouling, whereas B. violaceus was likely introduced as a ‘fellow traveler’ in the shellfish aquaculture trade.



Similar content being viewed by others
References
Avise JC, Arnold J, Ball RM, Bermingham E, Lamb T, Neigel JE, Reeb CA, Saunders NC (1987) Intraspecific phylogeography: the mitochondrial DNA bridge between population genetics and systematics. Annu Rev Ecol Syst 18:489–522
BCMAL (2007) Shellfish aquaculture: current statistics. British Columbia Ministry of Aquaculture and Lands, BC, 108 pp
Ben-Shlomo R, Paz G, Rinkevich B (2006) Postglacial-period and recent invasions shape the population genetics of botryllid ascidians along European Atlantic coasts. Ecosystems 9:1118–1127
Berril NJ (1950) The Tunicata, with an account of British species. Ray Society, London, iii+354pp
Brunetti R, Beghi L, Bressan M, Marin MG (1980) Combined effects of temperature and salinity on colonies of Botryllus schlosseri and Botrylloides leachi (Ascidiacea) from the Venetian Lagoon. Mar Ecol Prog Ser 2:303–314
Carlton JT (1989) Man’s role in changing the face of the ocean: biological invasions and implications for conservation of near-shore environments. Conserv Biol 3:265–266
Carlton JT (2005) Setting ascidian invasions on the global stage. International Invasive Sea Squirt Conference. Woods Hole Oceanographic Institution, Woods Hole
Carlton JT, Geller JB (1993) Ecological roulette—the global transport of nonindigenous marine organisms. Science 261:78–82
Carver CE, Mallet AL, Vercaemer B (2006) Biological synopsis of the colonial tunicates, Botryllus schlosseri and Botrylloides violaceus. Canadian Manuscript Report of Fisheries and Aquatic Sciences 2747: v+42p
Castilla JC, Guinez R, Caro AU, Ortiz V (2004) Invasion of a rocky intertidal shore by the tunicate Pyura praeputialis in the Bay of Antofagasta, Chile. Proc Natl Acad Sci 101:8517–8524
Clement M, Posada D, Crandall KA (2000) TCS: a computer program to estimate gene genealogies. Mol Ecol 9:1657–1659
Cohen AN, Carlton JT (1995) Biological study. Nonindigenous aquatic species in a United States estuary: a case study of the biological invasions of the San Francisco Bay and Delta. A report for the US Fish and Wildlife Service and National Sea Grant College Program. National Technical Information Service, Springfield, p 246
Colautti RI, MacIsaac HJ (2004) A neutral terminology to define ‘invasive’ species. Divers Distrib 10:135–141
Colautti RI, Manca M, Viljanen M, Ketelaars HAM, Burgi H, MacIsaac HJ, Heath DD (2005) Invasion genetics of the Eurasian spiny waterflea: evidence for bottlenecks and gene flow using microsatellites. Mol Ecol 14:1869–1879
Coutts ADM, Dodgshun TJ (2007) The nature and extent of organisms in vessel sea-chests: a protected mechanism for marine bioinvasions. Mar Pollut Bull 54:875–886
Crandall KA (1996) Multiple interspecies transmissions of human and simian T-cell leukemia/lymphoma virus type I sequences. Mol Biol Evol 13:115–131
Cristescu MEA, Hebert PDN, Witt JDS, MacIsaac HJ, Grigrovich IA (2001) An invasion history for Cercopagis pengoi based on mitochondrial gene sequences. Limnol Oceanogr 46:224–229
Deangelis MM, Wang DG, Hawkins TL (1995) Solid-phase reversible immobilization for the isolation of PCR products. Nucleic Acids Res 23:4742–4743
Dijkstra J, Harris LG, Westerman E (2007) Distribution and long-term temporal patterns of four invasive colonial ascidians in the Gulf of Maine. J Exp Mar Biol Ecol 342:61–68
Drake JM, Lodge DM (2004) Global hot spots of biological invasions: evaluating options for ballast-water management. Proc R Soc Lond Ser B Biol Sci 271:575–580
Dupont L, Viard F, Dowell MJ, Wood C, Bishop JDD (2009) Fine- and regional-scale genetic structure of the exotic ascidian Styela clava (Tunicata) in southwest England, 50 years after its introduction. Mol Ecol 18:442–453
Elphinstone MS, Hinten GN, Anderson MJ, Nock CJ (2003) An inexpensive and high-throughput procedure to extract and purify total genomic DNA for population studies. Mol Ecol Notes 3:317–320
Epelbaum A, Herborg LM, Therriault TW, Pearce CM (2009) Temperature and salinity effects on growth, survival, reproduction, and potential distribution of two non-indigenous botryllid ascidians in British Columbia. J Exp Mar Biol Ecol 369:43–52
Excoffier L, Smouse PE, Quattro JM (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131:479–491
Excoffier L, Laval G, Schneider S (2005) Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evol Bioinform Online 1:47–50
Fay RC, Vallee JA (1979) A survey of the littoral and sublittoral ascidians of southern California, including the Channel Islands. Bull South Calif Acad Sci 70:114–124
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Frankham R (2005) Stress and adaptation in conservation genetics. J Evol Biol 18:750–755
Fu Y-X (1997) Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 147:915–925
Gittenberger A (2007) Recent population expansions of non-native ascidians in the Netherlands. J Exp Mar Biol Ecol 342:122–126
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704
Holland BS (2000) Genetics of marine bioinvasions. Hydrobiologia 420:63–71
Jensen JL, Bohonak AJ, Kelley ST (2005) Isolation by distance, web service. BMC Genet 6:13
Jeschke JM, Strayer DL (2005) Invasion success of vertebrates in Europe and North America. Proc Natl Acad Sci 102:7198–7202
Keane T, Creevey C, Pentony M, Naughton T, Mclnerney J (2006) Assessment of methods for amino acid matrix selection and their use on empirical data shows that ad hoc assumptions for choice of matrix are not justified. BMC Evol Biol 6:29
Kolar CS, Lodge DM (2001) Progress in invasion biology: predicting invaders. Trends Ecol Evol 16:199–204
Kolbe JJ, Glor RE, Schettino LRG, Lara AC, Larson A, Losos JB (2004) Genetic variation increases during biological invasion by a Cuban lizard. Nature 431:177–181
Lambert G (2005) Ecology and natural history of the protochordates. Can J Zool 83:34–50
Lambert G (2007) Invasive sea squirts: a growing global problem. J Exp Mar Biol Ecol 342:3–4
Lambert CC, Lambert G (1998) Non-indigenous ascidians in southern California harbors and marinas. Mar Biol 130:675–688
Lambert CC, Lambert G (2003) Persistence and differential distribution of nonindigenous ascidians in harbors of the Southern California Bight. Mar Ecol Prog Ser 259:145–161
LeBlanc N, Davidson J, Tremblay R, McNiven M, Landry T (2007) The effect of anti-fouling treatments for the clubbed tunicate on the blue mussel, Mytilus edulis. Aquaculture 264:205–213
Lee CE (2002) Evolutionary genetics of invasive species. Trends Ecol Evol 17:386–391
LeGresley M, Martin J, McCurdy P, Thorpe B, Chang B (2008) Non-indigenous tunicate species in the Bay of Fundy, eastern Canada. ICES J Mar Sci 65:770–774
Lejeusne C, Chevaldonné P (2006) Brooding crustaceans in a highly fragmented habitat: the genetic structure of Mediterranean marine cave-dwelling mysid populations. Mol Ecol 15:4123–4140
Locke A, Hanson JM, Ellis KM, Thompson J, Rochette R (2007) Invasion of the southern Gulf of St. Lawrence by the clubbed tunicate (Styela clava Herdman): potential mechanisms for invasions of Prince Edward Island estuaries. J Exp Mar Biol Ecol 342:69–77
Lockwood JL, Cassey P, Blackburn T (2005) The role of propagule pressure in explaining species invasions. Trends Ecol Evol 20:223–228
Lopez-Legentil S, Turon X, Planes S (2006) Genetic structure of the star sea squirt, Botryllus schlosseri, introduced in southern European harbours. Mol Ecol 15:3957–3967
May GE, Gelembiuk GW, Panov VE, Orlova MI, Lee CE (2006) Molecular ecology of zebra mussel invasions. Mol Ecol 15:1021–1031
McCarthy A, Osman RW, Whitlatch RB (2007) Effects of temperature on growth rates of colonial ascidians: a comparison of Didemnum sp. to Botryllus schlosseri and Botrylloides violaceus. J Exp Mar Biol Ecol 342:172–174
Meenakshi VK, Senthamarai S (2006) First report on two species of ascidians to represent the genus Botryllus Gaertner, 1774 from Indian waters. J Mar Biol Assoc India 48:100–102
Muirhead J, Gray D, Kelly D, Ellis S, Heath D, MacIsaac H (2008) Identifying the source of species invasions: sampling intensity vs. genetic diversity. Mol Ecol 17:431–449
Nei M (1987) Molecular evolutionary genetics. Columbia University Press, New York
Pallas PS (1766) Elenchus zoophytorum sistens generum adumbrationes generaliores et specierum cognitarum succinctas descriptiones cum selectis auctorum synomymis. Varrentrapp, F, Frankfurt, p 539
Panchal M, Beaumont MA (2007) The automation and evaluation of nested clade phylogeographic analysis. Evolution 61:1466–1480
Paz G, Douek J, Mo CQ, Goren M, Rinkevich B (2003) Genetic structure of Botryllus schlosseri (Tunicata) populations from the Mediterranean coast of Israel. Mar Ecol Prog Ser 250:153–162
Perez-Portela R, Turon X (2008) Cryptic divergence and strong population structure in the colonial invertebrate Pycnoclavella communis (Ascidiacea) inferred from molecular data. Zoology 111:163–178
Petit RJ (2007) The coup de grâce for the nested clade phylogeographic analysis? Mol Ecol 17:516–518
Pfenninger M, Posada D (2002) Phylogeographic history of the snail Candidula unifasciata (Helicellinae, Stylommatophora): fragmentation, corridor migration, and secondary contact. Evolution 56:1776–1788
Posada D, Crandall KA, Templeton AR (2000) GeoDis: a program for the cladistic nested analysis of the geographical distribution of genetic haplotypes. Mol Ecol 9:487–488
Ramos-Onsins SE, Rozas J (2002) Statistical properties of new neutrality tests against population growth. Mol Biol Evol 19:2092–2100
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Rius M, Pascual M, Turon X (2008) Phylogeography of the widespread marine invader Microcosmus squamiger (Ascidiacea) reveals high genetic diversity of introduced populations and non-independent colonizations. Divers Distrib 14:818–828
Rius M, Pineda MC, Turon X (2009) Population dynamics and life cycle of the introduced ascidian Microcosmus squamiger in the Mediterranean Sea. Biol Invasions 11:2181–2194
Rogers AD, Harpending H (1992) Population growth makes waves in the distribution of pairwise genetic differences. Mol Biol Evol 9:552–569
Roman J (2006) Diluting the founder effect: cryptic invasions expand a marine invader’s range. Proc R Soc B Biol Sci 273:2453–2459
Roman J, Darling JA (2007) Paradox lost: genetic diversity and the success of aquatic invasions. Trends Ecol Evol 22:454–464
Rozas J, Sanchez-DelBarrio JC, Messeguer X, Rozas R (2003) DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 19:2496–2497
Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, Cabin RJ, Cohen JE, Ellstrand NC, McCauley DE, O’Neil P, Parker IM, Thompson JN, Weller SG (2001) The population biology of invasive species. Annu Rev Ecol Syst 32:305–332
Schneider S, Excoffier L (1999) Estimation of past demographic parameters from the distribution of pairwise differences when the mutation rates vary among sites: application to human mitochondrial. Genetics 152:1079–1089
Stoner DS, Ben-Shlomo R, Rinkevich B, Weissman IL (2002) Genetic variability of Botryllus schlosseri invasions to the east and west coasts of the USA. Mar Ecol Prog Ser 243:93–100
Suarez AV, Tsutsui ND, Holway DA (1999) Behavioral and genetic differentiation between native and introduced populations of the Argentine ant. Biol Invasions 1:43–53
Swofford DL (2001) PAUP*: phylogenetic analysis using parsimony (*and others methods). Sinauer, Sunderland
Templeton AR (2004) Statistical phylogeography: methods of evaluating and minimizing inference errors. Mol Ecol 13:789–809
Templeton A (2008) Nested clade analysis: an extensively validated method for strong phylogeographic inference. Mol Ecol 17:1877–1880
Templeton AR (2009) Statistical hypothesis testing in intraspecific phylogeography: nested clade phylogeographical analysis vs. approximate Bayesian computation. Mol Ecol 18:319–331
Templeton AR, Boerwinkle E, Sing CF (1987) A cladistic analysis of phenotypic associations with haplotypes inferred from restriction endonuclease mapping. I. Basic theory and an analysis of alcohol dehydrogenase activity in Drosophila. Genetics 117:343–351
Valentine PC, Collie JS, Reid RN, Asch RG, Guida VG, Blackwood DS (2007) The occurrence of the colonial ascidian Didemnum sp on Georges Bank gravel habitat—ecological observations and potential effects on groundfish and scallop fisheries. J Exp Mar Biol Ecol 342:179–181
Acknowledgments
We acknowledge our Canadian Aquatic Invasive Species Network (CAISN) colleagues G. Arsenault, J. Davidson, J. Hill, and A. Ramsay as well as our global colleagues T. Bolton, P. Chevaldonné, R. Graille, E. Grey, A. Izquierdo-Munoz, B. de Ligondes, S. Lopez-Legentil, J.-M. Nicolas, M. Ruis-Viladomiu, Y. Saito, X. Turon, and B. Vercaemer, who have so generously provided tunicate samples. D. Heath, S. Xu and A. Zhan provided valuable comments on an early draft of the manuscript. We also thank A. Adebayo, K. Laroche and R. Hepburn for assisting with lab work. This work was supported by CAISN, Fisheries and Oceans Canada and by NSERC Discovery grants to MEC and HJM.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lejeusne, C., Bock, D.G., Therriault, T.W. et al. Comparative phylogeography of two colonial ascidians reveals contrasting invasion histories in North America. Biol Invasions 13, 635–650 (2011). https://doi.org/10.1007/s10530-010-9854-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-010-9854-0