Abstract
Objective
To evaluate an analog library of betaine-type cellular metabolites, which are naturally found in polar fish for survival in subzero temperatures, for preventing denaturation of enzymes during freezing.
Results
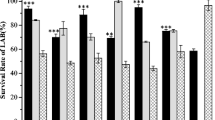
Comparison of the cryoprotective ability of reported cryoprotectants, such as dimethylsulfoxide, glycerol, ectoine, hydroxyectoine, and trehalose, with betaine-type analogs using α-glucosidase revealed that analogs introducing C3–C6 alkyl chains into an ammonium cation retained 20 % higher activity than the control cryoprotectants at the same concentration. In particular, the analog possessing triplicate n-butyl chains showed a profound effect. It allowed retention of enzyme activity to 95 % even after 100 freeze–thaw cycles, while addition of the control cryoprotectants decreased the activity to 10–20 %. The cryoprotective ability of betaine-type analogs can be applied not only to α-glucosidase but also other enzymes such as β-glucosidase, alkaline phosphatase, lactose dehydrogenase, sulfatase, and horseradish peroxidase.
Conclusion
Synthetic betaine-type metabolite analogs possess practicable cryoprotective ability for various enzymes, and are considerably superior to previously reported cryoprotectants.





Similar content being viewed by others
References
Arakawa T, Carpenter JF, Kita YA, Crowe JH (1990) The basis for toxicity of certain cryoprotectants: a hypothesis. Cryobiology 27:401–415
Carpenter JF, Crowe JH (1988) The mechanism of cryoprotection of proteins by solutes. Cryobiology 25:244–255
Crowe JH, Carpenter JF, Crowe LM, Anchordoguy TJ (1990) Are freezing and dehydration similar stress vectors? A comparison of modes of interaction of stabilizing solutes with biomolecules. Cryobiology 27:219–231
Deguchi E, Koumoto K (2011) Cellular zwitterionic metabolite analogs simultaneously enhance reaction rate, thermostability, salt tolerance, and substrate specificity of α-glucosidase. Bioorg Med Chem 19:3128–3134
Fahy GM, Levy DI, Ali SE (1987) Some emerging principles underlying the physical properties, biological actions, and utility of vitrification solutions. Cryobiology 24:196–213
Gallati H (1979) Horseradish peroxidase: a study of the kinetics and the determination of optimal reaction conditions, using hydrogen peroxide and 2,2′-azinobis 3-ethylbenzthiazoline-6-sulfonic acid (ABTS) as substrates. J Clin Chem Clin Biochem 17:1–7
Kim DE, Kim KH, Bae YJ, Lee JH, Jang YH, Nam SW (2005) Purification and characterization of the recombinant arylsulfatase cloned from Pseudoalteromonas carrageenovora. Protein Express Purif 39:107–115
Koumoto K, Ochiai H, Sugimoto N (2008) Structural effect of synthetic zwitterionic cosolutes on the stability of DNA duplexes. Tetrahedron 64:168–174
Lippert K, Galinski EA (1992) Enzyme stabilization by ectoine-type compatible solutes: protection against heating, freezing and drying. Appl Microbiol Biotechnol 37:61–65
Lyer PV, Ananthanarayan L (2008) Enzyme stability and stabilization—aqueous and non-aqueous environment. Process Biochem 43:1019–1032
Nakagawa Y, Sehata S, Fujii S, Yamamoto H, Tsuda A, Koumoto K (2014) Mechanistic study on the facilitation of enzymatic hydrolysis by α-glucosidase in the presence of betaine-type metabolite analogs. Tetrahedron 70:5895–5903
Nardid OA, Rozanova ED, Naumenko EI (2014) Freeze–thawing induced structural and functional changes in glucose oxidase. Cryo Lett 35:114–118
Nordlie FG (2009) Environmental influences on regulation of blood plasma/serum components in teleost fishes: a review. Rev Fish Biol Fisher 19:481–564
Schobert B (1977) Is there an osmotic regulatory mechanism in algae and higher plants? J Theor Biol 68:17–26
Uemitsu N, Ohashi H, Matsumiya H (1975) Nuclear magnetic resonance investigation of the state of water in protein solution. J Biochem 78:229–234
Yancey PH (2005) Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol 208:2819–2830
Supporting information
Supplementary Fig. 1—Plot of remaining activity of α-GLU in the enzyme stock solution as a function of freeze–thaw cycles. Cryopreservation conditions: [α-GLU] = 25 µg/ml, [phosphate buffer (pH 7.0)] = 100 mM, freeze–thaw cycles (fast freezing) = 0–50 times. Error bars represent the standard deviation of five measurements.
Supplementary Fig. 2—Plots of remaining activities of α-GLU in the stock solution as a function of concentration of analogs 7–14 after 12 freeze–thaw cycles. Cryopreservation conditions: [α-GLU] = 25 µg/ml, [phosphate buffer (pH 7.0)] = 100 mM, [analogs] = 0–1000 mM, freeze–thaw cycles (fast freezing) = 12 times. Error bars represent the standard deviation of five measurements.
Supplementary Fig. 3—Plots of remaining activities of LDH, ALP, HRP, β-GLU, and SUL in the enzyme stock solutions as a function of freeze–thaw cycles (fast freezing) (0–50 times). Cryopreservation conditions: α-GLU: [α-GLU] = 25 µg/ml, [phosphate buffer (pH 7.0)] = 100 mM, [analog 4] = 300 mM, β-GLU: [β-GLU] = 50 µg/ml, [phosphate buffer (pH7.0)] = 100 mM, [analog 4] = 300 mM, ALP: [ALP] = 50 µg/ml, [Tris buffer (pH 7.5)] = 100 mM, [analog 4] = 300 mM, SUL: [SUL] = 625 µg/ml, [phosphate buffer (pH 7.0)] = 100 mM, [analog 4] = 300 mM, LDH: [LDH] = 50 µg/ml, [Tris buffer (pH 7.5)] = 80 mM, [analog 4] = 300 mM, HRP: [HRP] = 1.0 µg/ml, [citric acid buffer (pH 4.2)] = 10 mM, [analog 4] = 300 mM. Error bars represent the standard deviation of five measurements.
Supplementary Fig. 4—Comparison of circular dichroisim (CD) spectra of α-GLU in the (a) absence and (b) presence of analog 4 before (solid line) and after 12 freeze–thaw cycles (broken line). Conditions: [α-GLU] = 0.5 mg/ml, [phosphate buffer (pH 7.0)] = 100 mM, [analog 4] = 0 or 300 mM at 30°C, freeze–thaw cycles (fast freezing) = 0 (solid line) or 12 times (broken line).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nakagawa, Y., Sota, M. & Koumoto, K. Cryoprotective ability of betaine-type metabolite analogs during freezing denaturation of enzymes. Biotechnol Lett 37, 1607–1613 (2015). https://doi.org/10.1007/s10529-015-1841-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-015-1841-1