Abstract
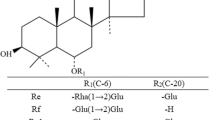
β-Glucosidase from Thermus thermophilus has specific hydrolytic activity for the outer glucose at the C-20 position in protopanaxadiol-type ginsenosides without hydrolysis of the inner glucose. The hydrolytic activity of the enzyme for gypenoside XVII was optimal at pH 6.5 and 90 °C, with a half-life of 1 h with 3 g enzyme l−1 and 4 g gypenoside XVII l−1. Under the optimized conditions, the enzyme converted the substrate gypenoside XVII to ginsenoside F2 with a molar yield of 100 % and a productivity of 4 g l−1 h−1. The conversion yield and productivity of ginsenoside F2 are the highest reported thus far among enzymatic transformations.





Similar content being viewed by others
References
Bae EA, Choo MK, Park EK, Park SY, Shin HY, Kim DH (2002) Metabolism of ginsenoside Rc by human intestinal bacteria and its related antiallergic activity. Biol Pharm Bull 25:743–747
Cheng LQ, Kim MK, Lee JW, Lee YJ, Yang DC (2006) Conversion of major ginsenoside Rb1 to ginsenoside F2 by Caulobacter leidyia. Biotechnol Lett 28:1121–1127
Cheng LQ, Na JR, Kim MK, Bang MH, Yang DC (2007) Microbial conversion of ginsenoside Rb1 to minor ginsenoside F2 and gypenoside XVII by Intrasporangium sp. GS603 isolated from soil. J Microbiol Biotechnol 17:1937–1943
Cho WC, Chung WS, Lee SK, Leung AW, Cheng CH, Yue KK (2006) Ginsenoside Re of Panax ginseng possesses significant antioxidant and antihyperlipidemic efficacies in streptozotocin-induced diabetic rats. Eur J Pharmacol 550:173–179
Hong H, Cui CH, Kim JK, Jin FX, Kim SC, Im WT (2012) Enzymatic biotransformation of ginsenoside Rb1 and gypenoside XVII into ginsenosides Rd and F2 by recombinant β-glucosidase from Flavobacterium johnsoniae. J Ginseng Res 36:418–424
Huang C, Wang G, Li H, Xie H, Sun J, Lv H, Lv T (2006) Sensitive and selective liquid chromatography–electrospray ionisation–mass spectrometry analysis of astragaloside-IV in rat plasma. J Pharm Biomed Anal 40:788–793
Lee SY, Kim GT, Roh SH, Song JS, Kim HJ, Hong SS, Kwon SW, Park JH (2009) Proteome changes related to the anti-cancer activity of HT29 cells by the treatment of ginsenoside Rd. Pharmazie 64:242–247
Lee GW, Kim KR, Oh DK (2012) Production of rare ginsenosides (compound Mc, compound Y and aglycon protopanaxadiol) by β-glucosidase from Dictyoglomus turgidum that hydrolyzes β-linked, but not α-linked, sugars in ginsenosides. Biotechnol Lett 34:1679–1686
Andreea Neculai M, Ivanov D, Bernards MA (2009) Partial purification and characterization of three ginsenoside-metabolizing β-glucosidases from Pythium irregulare. Phytochemistry 70:1948–1957
Quan LH, Piao JY, Min JW, Kim HB, Kim SR, Yang DU, Yang DC (2011) Biotransformation of ginsenoside Rb1 to prosapogenins, gypenoside XVII, ginsenoside Rd, ginsenoside F2, and compound K by Leuconostoc mesenteroides DC102. J Ginseng Res 35:344–351
Quan LH, Kim YJ, Li GH, Choi KT, Yang DC (2013) Microbial transformation of ginsenoside Rb1 to compound K by Lactobacillus paralimentarius. World J Microbiol Biotechnol 29:1001–1007
Shin KC, Oh DK (2013) Characterization of a novel recombinant β-glucosidase from Sphingopyxis alaskensis that specifically hydrolyzes the outer glucose at the C-3 position in protopanaxadiol-type ginsenosides. J Biotechnol. doi:10.1016/j.jbiotec.2013.11.026
Shin JY, Lee JM, Shin HS, Park SY, Yang JE, Cho SK, Yi TH (2012) Anti-cancer effect of ginsenoside F2 against Glioblastoma Multiforme in xenograft model in SD rats. J Ginseng Res 36:86–92
Wang L, Liu QM, Sung BH, An DS, Lee HG, Kim SG, Kim SC, Lee ST, Im WT (2011a) Bioconversion of ginsenosides Rb1, Rb2, Rc and Rd by novel β-glucosidase hydrolyzing outer 3-O glycoside from Sphingomonas sp. 2F2: cloning, expression, and enzyme characterization. J Biotechnol 156:125–133
Wang L, Zhang Y, Chen J, Li S, Wang Y, Hu L, Wu Y (2011b) Immunosuppressive effects of ginsenoside-Rd on skin allograft rejection in rats. J Surg Res 176:267–274
Xu QF, Fang XL, Chen DF (2003) Pharmacokinetics and bioavailability of ginsenoside Rb1 and Rg1 from Panax notoginseng in rats. J Ethnopharmacol 84:187–192
Yoshikawa M, Morikawa T, Kashima Y, Ninomiya K, Matsuda H (2003) Structures of new dammarane-type triterpene saponins from the flower buds of Panax notoginseng and hepatoprotective effects of principal ginseng saponins. J Nat Prod 66:922–927
Acknowledgments
This work was supported by the Basic Research Lab Program (No. 2010-0019306), the National Research Foundation, the Ministry of Education, Science and Technology, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shin, KC., Seo, MJ., Oh, HJ. et al. Highly selective hydrolysis for the outer glucose at the C-20 position in ginsenosides by β-glucosidase from Thermus thermophilus and its application to the production of ginsenoside F2 from gypenoside XVII. Biotechnol Lett 36, 1287–1293 (2014). https://doi.org/10.1007/s10529-014-1472-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-014-1472-y