Abstract
A membrane (M), protein-based ELISA was developed to detect porcine epidemic diarrhea virus (PEDV). The M gene of PEDV was expressed in Escherichia coli. The purified recombinant M protein was used to immunize rabbits to generate a polyclonal antibody. Immunofluorescence analysis indicated that the anti-PEDV-M antibody reacted with PEDV-infected cells. The antibody was utilized to develop an indirect ELISA to detect PEDV. Other viruses, porcine transmissible gastroenteritis coronavirus, avian infectious bronchitis coronavirus, porcine reproductive and respiratory syndrome virus, classic swine fever virus and porcine pseudorabies virus, were unreactive.
Similar content being viewed by others
Introduction
Porcine epidemic diarrhea (PED) is contagious, enteric disease. The major clinical signs of PED include vomiting, diarrhea and dehydration. The mortality in infected neonatal piglets less than 5 days old may reach 100% (Kim and Chae 2000). The causative agent of PED is porcine epidemic diarrhea virus (PEDV), which belongs to the order Nidovirales, family Coronaviridae, genus Coronavirus. PEDV was first recognized in Europe in 1977 (Pensaert and Debouck 1978). Several outbreaks of PED have been reported mostly in Europe and East Asia (Pensaert and Sang-Geon 2006).
Coronaviruses, including PEDV, are enveloped, single-stranded RNA viruses and contain four major structural proteins: spike (S) (180–220 kDa), envelope (E), membrane (M) (27–32 kDa) and nucleocapsid (N) (55–58 kDa) (Murphy et al. 1999; Ren et al. 2008). The M protein plays an important role in the assembly process of viral nucleocapsid and membrane. The M glycoprotein can neutralize anti-M antibody in the present of complement and stimulate the production of interferon (Laude et al. 1992). The M protein is abundant among the structural proteins of PEDV. In addition, the nucleotide sequence of the M gene possess low homology (approx. 50%) with other coronaviruses, but is highly conserved among different PEDV strains (Kim and Chae 2000). Therefore, the M protein may be used as a candidate diagnostic reagent for determining PEDV infection.
In this study, we have expressed the M gene of PEDV and generated an anti-M polyclonal antibody via animal inoculation. Analysis on the activities of the M protein and the antibody indicated that both proteins were biologically active. The antibody was able to discriminate PEDV from other viruses selected.
Materials and methods
Construction of expression plasmid, pET-PEDV-M
A recombinant plasmid pEasy T-M containing the full-length M gene of PEDV was used as a template to amplify a signal peptide sequence-deleted M gene using a sense primer: 5′-GGGCGAATTCATGACTCTTGAAATTTCATGTTAT-3′ and a reverse primer: 5′-CCGGGTCGACTTTTATAACAGCTGTGGCATCTAA-3′. Both primers contained EcoRI and XhoI sites (underline parts), respectively. The PCR parameters included 95°C for 5 min, 30 cycles of 95°C for 1 min, 55.6°C for 1 min, 72°C for 1 min and a final extension of 72°C for 8 min. The PCR product was cloned into the same sites of a prokaryotic expression vector, pET-30a (Novagen, USA), resulting a recombinant plasmid pET-PEDV-M. The DNA plasmid was purified using a purification kit (KeyGen Biotech, China) prior to DNA sequencing.
Expression and purification of the protein of interest
The pET-PEDV-M was transformed into host cells [E. coli BL21(DE3) pLysS] and the protein expression was analyzed according to a recent reference with modifications (Liu et al. 2009). Briefly, the bacteria harboring the pET-PEDV-M were cultured in Luria-Bertani (LB) medium at 37°C with shaking until the OD600 reached 0.5. Then, IPTG was added to 0.5 or 1 mM to induce protein expression at 37 or 30°C for 6 h. Empty vector-transformed bacteria were used as control. Expression amount of the M protein in the total bacterial protein was analyzed using software. The bacterial suspension (20 ml) was centrifuged; the cells were re-suspended in TE buffer (50 mM Tris/HCl and 1 mM EDTA, pH 8.0), mixed with lysozyme (100 μg/l) at room temperature for 30 min, then was sonicated on ice for 30 min and finally centrifuged at 10,000×g for 20 min. The supernatant and pellets were mixed with SDS-loading buffer, subjected to 12% (v/v) SDS-PAGE and the gels were stained with Coomassie brilliant blue. The gel purification and re-naturation of fusion protein by dialysis were performed according to Liu et al. (2009). The protein of interest was designated as PEDV-M.
Preparation and titration of anti-M protein polyclonal antibody
To generate a specific polyclonal antibody against the PEDV-M protein, two New Zealand rabbits were immunized with the purified PEDV-M protein (2 mg/ml) emulsified with Freund’s complete adjuvant via subcutaneous injection (1 ml/each rabbit). Two weeks later, the rabbits were injected with the same antigen (0.5 ml) mixed with Freund’s incomplete adjuvant. Subsequent injections were made every week for 10 weeks. Blood of the immunized rabbits was collected from neck artery and the anti-M antibody was isolated. The antibody titer was determined using ELISA. Briefly, ELISA plates were coated with the PEDV-M protein (1 μg/well) at 4°C and held overnight in 50 mM Na2CO3 /NaHCO3 buffer, pH 8.6. The next day, the wells were blocked with 5% (w/v) non-fat dry milk in PBS/0.05% Tween 20 (PBST) at 37°C for 2 h, after washing three times with PBST. Then the wells were incubated with serially diluted polyclonal antibody at 37°C for 1 h. Serum from a non-immunized rabbit was used as control. The wells were incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (1:5,000 diluted in PBS) at 37°C for 1 h followed by addition of o-phenylenediamine dihydrochloride (OPD) for 10 min. The reaction was stopped using 2 M H2SO4 and the A450 value was read using an ELISA plate reader.
Western blot
E. coli expressing either the PEDV-M protein or empty vector was induced by IPTG and the lysates were subjected to 12% (v/v) SDS-PAGE. The proteins were electronically transferred to a nitrocellulose membrane which was blocked overnight at 4°C using 5% (w/v) non-fat dry milk in PBST buffer and then incubated with anti-M polyclonal antibody (1:400 dilution in PBST) at room temperature for 2 h. After the membrane was washed three times with PBST, they were incubated with HRP-conjugated goat anti-rabbit IgG (1:2,000 dilution in PBST) for another 1 h. The protein bands were visualized using OPD.
Immunofluorescence
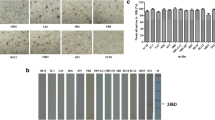
African green monkey kidney cell line, Vero cells (ATCC CCL-81) were cultured in Dulbecco’s modified essential medium (DMEM) supplemented with 5% (v/v) fetal bovine serum at 37°C. The next day, the cells were infected with PEDV isolate HLJBY at 37°C for 48–72 h. Immunofluorescence assays were performed (see Ren et al. 2006; Sui et al. 2010) with modifications. Briefly, the cells were fixed 4% (w/v) formalin in PBS for 20 min and then were quenched with 0.1 M glycine in PBS. Then they were incubated with the anti-M antibody at 1:100 in 1% BSA at 37°C for 1 h. The cells were incubated with fluorescein isothiocyanate-labeled goat anti-rabbit IgG (Sigma), 1:200 diluted in 1% BSA for another 1 h in the dark. After washing three times with PBS, the fluorescence was observed under a fluorescence microscope.
ELISA
ELISA plates were coated with a panel of viruses (see Fig. 5). The coating concentration was 2 μg per well at 4°C overnight. The next day, the wells were blocked with 5% (w/v) non-fat dry milk-PBST at 37°C for 2 h. The anti-PEDV-M protein antibody (1:100 diluted in PBST) was added into the wells at 37°C for 1 h. After rinsing three times with PBST, the wells were incubated with HRP-conjugated goat anti-rabbit IgG (1:1,000 diluted in PBST) at 37°C for 1 h. OPD was added and incubated for 15 min after washing with PBST. The A450 was read using an ELISA plate reader.
Results
Construction and expression of the recombinant plasmid bearing PEDV-M gene
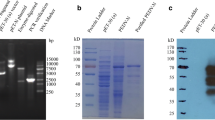
The signal peptide sequence-deleted PEDV-M gene was amplified using PCR and then cloned into the prokaryotic expression vector. The authenticity of the insert was confirmed by DNA sequencing. The cloned gene was identical to the reported M gene sequence of PEDV strain CV777 (GenBank accession number: AF353511). SDS-PAGE analysis indicated that expression of PEDV-M protein was detectable at 1 h post-IPTG induction (Fig. 1a). The protein was expressed as inclusion body in recombinant E. coli and its molecular weight was approx. 27 kDa as expected (Fig. 1b). The optimal induction condition for PEDV-M protein expression was 0.5 mM IPTG at 37°C. The expressed M protein was then purified (see Fig. 2).
Expression of PEDV-M protein in E. coli. The bacteria containing either PEDV-M gene or empty vector were induced by 0.5 mM IPTG at 30°C. The M protein expression was determined every hour using SDS-PAGE (a). Lane M: Protein molecular weight marker, Lane 1: Uninduced bacteria, Lanes 2, 4, 6, 8, 10, 12: Lysates of empty vector transformed bacteria at 1–6 h post-IPTG induction, Lanes 3, 5, 7, 9, 11: Expressed M protein at 1–5 h post-IPTG induction. The bacteria were lysed and centrifuged. The supernatant and pellets were subjected to SDS-PAGE (b). Lane M: Protein molecular weight marker, Lane 1: Expressed M protein at 6 h post-induction, Lane 2: Empty vector transformed bacterium control, Lanes 3 and 4: Supernatant and pellets of lysed bacteria containing the M gene at 6 h post-induction, respectively. Arrows indicate the position of the M protein with a molecular weight of approx. 27 kDa
Purification of PEDV-M protein. The expression of PEDV-M protein in E. coli was induced by 0.5 mM IPTG at 37°C for 4 h and the gel was stained with 0.25 M cold potassium chloride for 30 min followed by gel purification. The unpurified bacterial lysate and purified M protein were analyzed by SDS-PAGE. Lane M Protein molecular weight marker, Lane 1 Unpurified bacterial lysate, Lane 2 Purified M protein
Production and biological analysis of the anti-PEDV-M antibody
The purified M protein was used to immune rabbits for generating a polyclonal antibody. The antibody titer was 1:1012. To analyze the recognizing ability of the anti-PEDV-M antibody to the M protein, the antibody was used as primary antibody in a conventional Western blot. The polyclonal antibody reacted with the M protein (Fig. 3). The biological activity of the antibody was further analyzed using an immunofluorescence assay. Green fluorescence was detected in the PEDV-infected cells (Fig. 4).
Western blot analysis of PEDV-M protein. After the proteins from empty vector- or PEDV-M-transformed bacteria were transferred on a nitrocellulose membrane, a conventional immunoblotting was performed using anti-M antibody generated in this study as primary antibody. Their Western blot results are shown in lanes 1 and 2, respectively. Lane M Protein molecular weight marker
ELISA discriminating PEDV from other viruses
To investigate the utility of the antibody further, various viruses were used as coating antigens in indirect ELISA. Our results indicated that the anti-M antibody reacted with PEDV exclusively and no cross-reaction between the antibody and other viruses was detected (Fig. 5).
Discrimination of PEDV from other viruses in ELISA. The anti-PEDV-M antibody produced in this study was used as primary antibody in ELISA to detect a panel of viruses. Virus lysis solution was used as control buffer. A450 is shown in y axis. PEDV, transmissible gastroenteritis coronavirus (TGEV) infectious bronchitis coronavirus (IBV), porcine reproductive and respiratory syndrome virus (PRRSV), classic swine fever virus (CSFV), porcine pseudorabies virus (PrV) and buffer are indicated in x axis
Discussion
The first Chinese PEDV was isolated in 1980. Since then more PEDV infections have been reported in different areas and PED has been a serious threat to the pig industry in China. Effective diagnostic methods are required for surveillance of PED. The first purpose of the current study was to express PEDV-M gene. The expression of coronavirus M protein is difficult due to its sprout function. The viral M protein may cause damage to cell wall resulting in the growth inhibition (Kopecky and Lyles 2003). PEDV-M protein has been expressed in eukaryotic cells and a baculovirus system (Utiger et al. 1995). However, low expression and high costs may affect the application of the expressed protein. In contrast, the prokaryotic system is a time- and cost-efficient system for high-level protein production. Selection of a suitable prokaryotic system is important for foreign gene expression (Yin et al. 2007). We have already expressed several heterologous proteins, including glycoproteins, in prokaryotic systems and the bacterially-expressed proteins display excellent biological activities (Liu et al. 2009; Ren et al. 2010a, b).
High-level prokaryotic expression of envelope exterior of PEDV-M protein has been reported (Shenyang et al. 2007). In this study, we expressed full-length PEDV-M gene using a similar expression system. Availability of the mature M protein may allow us to identify more antigenic epitopes in this protein and make more functional analysis in the future. The M protein of PEDV has been identified by use of rabbit anti-peptide sera and transient expression of the M gene in Vero cells and by expression in the baculovirus system. The native M protein of PEDV is incorporated into virions, is N-glycosylated and with a molecular size of 27 kDa. In contrast, the M protein synthesized by recombinant baculoviruses migrates with an Mr of 23 kDa that is identical with the deglycosylated product of PEDV. M protein specified by the recombinant baculovirus is poorly, if at all, glycosylated (Utiger et al. 1995). In this study, the bacterially-expressed M protein is excluded from glycosylation and also migrates with an Mr of approx. 23 kDa (excluding the His-tag in the N-terminus). However, amount of the protein is enough for functional analysis and related experiments. The M protein was inoculated into rabbits to generate antibody. At different immunization intervals, we found that the titer of the anti-M antibody increased slowly, therefore, we had to prolong the immunization period (up to 3 months) to achieve a higher antibody titer. In our study, the sera collected from two immunized rabbits gave the similar antibody titer. Western blot and immunofluorescence assays showed that the antibody recognized the M protein and the virus-infected cells, indicating that the polyclonal antibody can be used for detection of PEDV.
Enteric infection caused by PEDV should be differentiated from other enteric viral diseases such as TGEV, a counterpart of the same group in the family Coronaviridae. They caused similar clinical signs and histological intestinal lesions, such as severe villus atrophy in the proximal and distal jejunum as well as in the ileum (Kim and Chae 2000). Herein porcine coronaviruses (TGEV and PEDV), avian coronavirus (IBV), porcine arterivirus (PRRSV), porcine pestivirus (CSFV) and porcine herpesvirus (PrV) were used as coating antigens in an indirect ELISA. The viruses have been confirmed by ELISA or PCR (data not shown). The results showed that the anti-M polyclonal antibody was specific to PEDV. These selected viruses are common pathogens in China and some of which may cause mixed infection with PEDV. The established ELISA described in this paper is valuable for differentiating infection between PEDV and other related viruses.
References
Kim O, Chae C (2000) In situ hybridization for the detection and localization of porcine epidemic diarrhea virus in the intestinal tissues from naturally infected piglets. Vet Pathol 37:62–67
Kopecky SA, Lyles DS (2003) Contrasting effects of matrix protein on apoptosis in HeLa and BHK cells infected with vesicular stomatitis virus are due to inhibition of host gene expression. J Virol 77:4658–4669
Laude H, Gelfi J, Lavenant L, Charley B (1992) Single amino acid changes in the viral glycoprotein M affect induction of alpha interferon by the coronavirus transmissible gastroenteritis virus. J Virol 66:743–749
Liu B, Li G, Sui X, Yin J, Wang H, Ren X (2009) Expression and functional analysis of porcine aminopeptidase N produced in prokaryotic expression system. J Biotechnol 141:91–96
Murphy FA, Gibbs EPJ, Horzinek MC, Studdert MJ (1999) Viral taxonomy and nomenclature. In: Mayo MA, Maniloff J, Desselberger U, Ball LA (eds) Veterinary virology. Academic Press, San Diego (CA), pp 23–42
Pensaert MB, Debouck P (1978) A new coronavirus-like particle associated with diarrhea in swine. Arch Virol 58:243–247
Pensaert MB, Sang-Geon YEO (2006) Porcine epidemic diarrhea. In: Straw BE, Zimmerman JJ, D’Allaire S, Taylor DJ (eds) Diseases of swine, 9th edn. Blackwell, Oxford, pp 367–372
Ren X, Glende J, Al-Falah M, de Vries V, Schwegmann-Wessels C, Qu X, Tan L, Tschernig T, Deng H, Naim HY, Herrler G (2006) Analysis of ACE2 in polarized epithelial cells: surface expression and function as receptor for severe acute respiratory syndrome-associated coronavirus. J Gen Virol 87:1691–1695
Ren X, Glende J, Yin J, Schwegmann-Wessels C, Herrler G (2008) Importance of cholesterol for infection of cells by transmissible gastroenteritis virus. Virus Res 137:220–224
Ren X, Wang M, Yin J, Li G (2010a) Phages harboring specific peptides to N protein of PRRSV distinguish the virus from other viruses. J Clin Microbiol 48:1875–1881
Ren X, Wang M, Yin J, Ren Y, Li G (2010b) Heterologous expression of fused genes encoding the glycoprotein 5 from PRRSV: a way for producing functional protein in prokaryotic microorganism. J Biotechnol 147:130–135
Shenyang G, Enhui Z, Baoxian L, Xinyuan Q, Lijie T, Junwei G, Yijing L (2007) High-level prokaryotic expression of envelope exterior of membrane protein of porcine epidemic diarrhea virus. Vet Microbiol 123:187–193
Sui X, Yin J, Ren X (2010) Antiviral effect of diammonium glycyrrhizinate and lithium chloride on cell infection by pseudorabies herpesvirus. Antiviral Res 85:346–353
Utiger A, Tobler K, Bridgen A, Ackermann M (1995) Identification of the membrane protein of porcine epidemic diarrhea virus. Virus Genes 10:137–148
Yin J, Li G, Ren X, Herrler G (2007) Select what you need: a comparative evaluation of the advantages and limitations of frequently used expression systems for foreign genes. J Biotechnol 127:335–347
Acknowledgments
Funding supported by Program for New Century Excellent Talents in Heilongjiang Provincial University (1155–NCET–005), Heilongjiang Provincial Science and Technology Department (ZJN0702-01) and National Natural Science Foundations of China (30700590; 30700591; 30972195) are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ren, X., Suo, S. & Jang, YS. Development of a porcine epidemic diarrhea virus M protein-based ELISA for virus detection. Biotechnol Lett 33, 215–220 (2011). https://doi.org/10.1007/s10529-010-0420-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-010-0420-8