Abstract
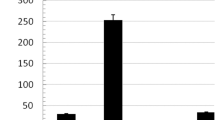
The Bacillus pumilus SG2 chitinase gene (ChiS) and its truncated form lacking chitin binding (ChBD) and fibronectin type III (FnIII) domains were transformed to Arabidopsis plants and the expression, functionality and antifungal activity of the recombinant proteins were investigated. Results showed that while the two enzyme forms showed almost equal hydrolytic activity toward colloidal chitin, they exhibited a significant difference in antifungal activity. Recombinant ChiS in plant protein extracts displayed a high inhibitory effect on spore germination and radial growth of hyphae in Alternaria brassicicola, Fusarium graminearum and Botrytis cinerea, while the activity of the truncated enzyme was strongly abolished. These findings demonstrate that ChBD and FnIII domains are not necessary for hydrolysis of colloidal chitin but play an important role in hydrolysis of chitin–glucan complex of fungal cell walls. Twenty microgram aliquots of protein extracts from ChiS transgenic lines displayed strong antifungal activity causing up to 80% decrease in fungal spore germination. This is the first report of a Bacillus pumilus chitinase expressed in plant system.


Similar content being viewed by others
References
Ahmadian G, Degrassi G, Venturi V, Zeigler DR, Soudi M, Zanguinejad P (2007) Bacillus pumilus SG2 isolated from saline conditions produces and secretes two chitinases. J Appl Microbiol 103:1081–1089
Ausubel FM, Brent R, Kingston RE, Moore DM, Seidman SG, Smith JA, Struhl K (1995) Current protocols in molecular biology. Wiley, Boston
Bechtold N, Ellis J, Pelletier G (1993) In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C R Acad Sci Paris Life Sci 316:1194–1199
Bishop JG, Dean AM, Mitchell-Olds T (2000) Rapid evolution in plant chitinases: molecular targets of selection in plant–pathogen coevolution. Proc Natl Acad Sci USA 97:5322–5327
Boller T, Gehri A, Mauch F, Vogeli U (1983) Chitinase in bean leaves: induction by ethylene, purification, properties, and possible function. Planta 157:22–31
Fung KL, Zhao KJ, He ZM, Chye ML (2002) Tobacco-expressed Brassica juncea chitinase BjCHI1 shows antifungal activity in vitro. Plant Mol Biol 50:283–294
Gidoni D, Gilbert D, Bond-Nutter D, Lee R, Bedbrook J, Dunsmuir P (1988) Expression of bacterial chitinase protein in tobacco leaves using two photosynthetic gene promoters. Mol Gen Genet 212:536–542
Grover A, Gowthaman R (2003) Strategies for development of fungus-resistant transgenic plants. Curr Sci 84:330–340
Hassan F, Meens J, Jacobsen HJ, Kieseckerd H (2009) A family 19 chitinase (Chit30) from Streptomyces olivaceoviridis ATCC 11238 expressed in transgenic pea affects the development of T. harzianum in vitro. J Biotechnol 143:302–308
Iseli B, Boller T, Neuhaus JM (1993) The N-terminal cysteine-rich domain of tobacco class I chitinase is essential for chitin biding but not for catalytic or antifungal activity. Plant Physiol 103:221–226
Itoh Y, Kawase T, Nikaidou N, Fukada H, Mitsutomi M, Watanabe T, Itoh Y (2002) Functional analysis of the chitin binding domain of a family 19 chitinase from Streptomyces griseus HUT6037: substrate-binding affinity and cis-dominant increase of antifungal function. Biosci Biotechnol Biochem 66:1084–1092
Itoh Y, Takahashi K, Takizawa H, Nikaidou N, Tanaka H, Nishihashi H, Watanabe T, Nishizawa Y (2003) Family 19 chitinase of Streptomyces griseus HUT6037 increases plant resistance to the fungal disease. Biosci Biotechnol Biochem 67:847–855
Kasprzewska A (2003) Plant chitinases: regulation and function. Cell Mol Biol Lett 8:809–824
Kuranda MJ, Robbins PW (1991) Chitinase is required for cell separation during growth of Saccharomyces cerevisiae. J Biol Chem 266:19758–19767
Lund P, Lee R, Dunsmuir P (1989) Bacterial chitinase is modified and secreted in transgenic tobacco. Plant Physiol 91:130–135
Moravcikova J, Libantova J, Heldak J, Salaj J, Bauer M, Matusikova I, Galova Z, Mlynarova L (2007) Stress-induced expression of cucumber chitinase and Nicotiana plumbaginifolia β-1,3-glucanase genes in transgenic potato plants. Acta Physiol Plant 29:133–141
Morimoto K, Karita S, Kimura T, Sakka K, Ohmiya K (1997) Cloning, sequencing, and expression of the gene encoding Clostridium paraputrificum chitinase ChiB and analysis of the functions of novel cadherin-like domains and a chitin-binding domain. J Bacteriol 179:7306–7314
Patil RS, Deshpande MV, Ghormade V (2000) Chitinolytic enzymes: an exploration. Enzyme Microb Technol 26:473–483
Punja ZK (2006) Recent developments toward achieving fungal disease resistance in transgenic plants. Can J Plant Pathol 28:298–308
Schlumbaum A, Mauch F, Vogeli U, Boller T (1986) Plant chitinases are potent inhibitors of fungal growth. Nature 324:365–367
Suarez V, Staehelin C, Arango R, Holtorf H, Hofsteenge J, Meins F (2001) Substrate specificity and antifungal activity of recombinant tobacco class I chitinases. Plant Mol Biol 45:609–618
Suslow TV, Matsubara D, Jones J, Lee R, Dunsmuir P (1988) Effect of expression of bacterial chitinase on tobacco susceptibility to leaf brown spot. Phytopathology 78:1556–1562
Watanabe T, Itoh Y, Yamada T, Hashimoto M, Sekine S, Tanaka H (1994) The roles of the C-terminal domain and type III domains of chitinase A1 from Bacillus circulans WL-12 in chitin degradation. J Bacteriol 176:4465–4472
Yamagami T, Funatsu G (1996) Limited proteolysis and reduction-carboxymethylation of rye seed chitinase-a: role of the chitin-binding domain in its chitinase action. Biosci Biotechnol Biochem 60:1081–1086
Acknowledgments
This research was supported by International Center for Genetic Engineering and Biotechnology (ICGEB, Italy) and National Institute for Genetic Engineering and Biotechnology (NIGEB, Iran).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dehestani, A., Kazemitabar, K., Ahmadian, G. et al. Chitinolytic and antifungal activity of a Bacillus pumilus chitinase expressed in Arabidopsis . Biotechnol Lett 32, 539–546 (2010). https://doi.org/10.1007/s10529-009-0192-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-009-0192-1