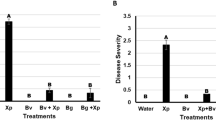
Abstract
To investigate the biocontrol effectiveness of the antibiotic producing bacterium, Pseudomonas aureofaciens 63–28 against the phytopathogen Rhizoctonia solani AG-4 on Petri plates and in soybean roots, growth response and induction of PR-proteins were estimated after inoculation with P. aureofaciens 63–28 (P), with R. solani AG-4 (R), or with P. aureofaciens 63–28 + R. solani AG-4 (P + R). P. aureofaciens 63–28 showed strong antifungal activity against R. solani AG-4 pathogens in Petri plates. Treatment with P. aureofaciens 63–28 alone increased the emergence rate, shoot fresh weight, shoot dry weight and root fresh weight at 7 days after inoculation, when compared to R. solani AG-4; P + R treatment showed similar effects. Peroxidase (POD) and β-1,3-glucanase activity of P. aureofaciens 63–28 treated roots increased by 41.1 and 49.9%, respectively, compared to control roots. POD was 26% greater in P + R treated roots than R. solani treated roots. Two POD isozymes (59 and 27 kDa) were strongly induced in P + R treated roots. The apparent molecular weight of chitinase from treated roots, as determined through SDS-PAGE separation and comparison with standards, was about 29 kDa. Five β-1,3-glucanase isozymes (80, 70, 50, 46 and 19 kDa) were observed in all treatments. These results suggest that inoculation of soybean plants with P. aureofaciens 63–28 elevates plant growth inhibition by R. solani AG-4 and activates PR-proteins, potentially through induction of systemic resistance mechanisms.




Similar content being viewed by others
References
Apostol I, Heinstein PF, Low PS (1989) Rapid stimulation of an oxidative burst during elicitation of cultured plant cells. Role in defense and signal transduction. Plant Physiol 90:109–116
Arlat M, Van Gijsegem F, Huet JC, Pernolletand JC, Boucher CA (1994) PopA1, a protein which induces a hypersensitivity-like response on specific Petunia genotypes, is secreted via the Hrp pathway of Pseudomonas solanacearum. EMBO J 13:543–553
Atlas RM (1995) Handbook of media for environmental microbiology. CRC Press, Boca Raton, FL
Baker KF, Snyder WC (1965) Ecology of soil-borne plant pathogens: prelude to biological control. Univ. of California Press, Berkeley
Benhamou N, Belanger PR, Paulitz TC (1996) Induction of differential host responses by Pseudomonas fluorescens in Ri T-DNA-transformed pea roots after challenge with Fusarium oxysporium f. sp. pisi and Phythium ultimum. Phytopathology 90:45–56
Boller T, Gehri A, Mauch F, Vögeli U (1983) Chitinase in bean leaves: induction by ethylene, purification, properties, and possible function. Planta 157:22–31
Bradford MM (1976) A rapid sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Caruso C, Chilosi G, Caporale C, Leonardi L, Bertini L, Magro P, Buonocore V (1999) Induction of pathogenesis-related proteins in germinating wheat seeds infected with Fusarium culmorum. Plant Sci 140:107–120
Chance B, Maehly AC (1955) Assay of catalases and peroxidases. Methods Enzymol 2:764–775
Conrath U, Domard A, Kauss H (1989) Chitosan-elicited synthesis of callose and of coumarin derivatives in parsley cell suspension cultures. Plant Cell Reptr 8:152–155
Dixon RA, Dey PM, Lamb CJ (1983) Phytoalexins: enzymology and molecular biology. Adv Enzymol 55:1–136
Gamard P, Sauriol F, Benhamou N, Belanger RR, Paulitz TC (1997) Novel butyrolactones with antifungal activity produced by Pseudomonas aureofaciens strain 63–28. J Antibiot 50:742–749
Jung WJ, Jin YL, Kim YC, Kim KY, Park RD, Kim TH (2004) Inoculation of Paenibacillus illinoisensis alleviates root mortality, activates of lignification-related enzymes, and induction of the isozymes in pepper plants infected by Phytophthora caisici. Biol Cont 30:645–652
Jung WJ, Jin YL, Kim KY, Park RD, Kim TH (2005) Changes in pathogenesis-related proteins in pepper plants with regard to biological control of phytopthora blight with Paenibacillus illinoisensis. BioControl 50:165–178
Keen NT, Yoshikawa M (1983) β-1,3-Endoglucanase from soybean releases elicitor-active carbohydrates from fungus cell walls. Plant Physiol 71: 460–465
Kerby K, Somerville SC (1992) Purification of an infection-related, extracellular peroxidase from barley. Plant Physiol 100:397–402
Lingappa Y, Lockwood JL (1962) Chitin media for selective isolation and culture of Actinomycetes. Phytopathology 52:317–323
Mauch F, Hadwiger LA, Boller T (1988) Antifungal hydrolases in pea tissue I. Purification and characterization if two chitinase and two β-glucanase differentially regulated during development and in response to fungal infection. Plant Physiol 87:325–333
Maurhofer M, Hase C, Meuwly P, Métraux JP, Défago G (1994) Induction of systemic resistance of tobacco to tobacco necrosis virus by the root-colonizing Pseudomonas fluorescens strain CHAO: influence of the gacA gene and of pyoverdine production. Phytopathology 84:139–146
Mellon JE (1991) Purification and characterization of isoperoxidases elicited by Aspergillus flavus in cotton ovule cultures. Plant Physiol 95:14–20
Mohammadi M, Kazemi H (2002) Changes in peroxidase and polyphenol oxidase activities in susceptible and resistant wheat heads inoculated with Fusarium graminearum and induced resistance. Plant Sci 162:491–498
M’Piga P, Belanger RR, Paulitz TC, Benhamou N (1997) Increased resistance to Fusarium oxysporum f. sp. radicis-lycopersiciin tomato plants treated with the endophytic bacterium Pseudomonas fluorescens strain 63–28. Physiol Mol Plant Path 50:301–320
Paulitz TC, Loper JL (1991) Lack of a role for fluorescent siderophore production in the biological control of Pythium damping-off by a strain of Pseudomonas putida. Phytopathology 81:930–935
Ramamoorthy V, Raguchander T, Samiyappan R (2002) Induction of defense-related proteins in tomato roots treated with Pseudomonas fluorescens Pf1 and Fusarium oxysporum f. sp. lycopersici. Plant Soil 239:55–68
SAS institute (2002) SAS/STAT User’s Guide, Version 9.1. Cary, NC, USA
Staehelin C, Granado J, Muller J, Wiemken A, Mellor RB, Felix G, Regenass M, Broughton WJ, Boiler T (1994) Perception of Rhizobium nodulation factors by tomato cells and inactivation by root chitinases. Proc Natl Acad Sci (USA) 91:2196–2200
Trudel J, Asselin A (1989) Detection of chitinase activity after polyacrylamide gel electrophoresis. Anal Biochem 178:362–366
Trudel J, Grenier J, Potvin C, Asselin A (1998) Several thaumatin-like proteins bind to β-1,3-glucans. Plant Physiol 118:1431–1438
Van Loon LC (1997) Induced resistance in plants and the role of pathogenesis-related proteins. Eur J Plant Pathol 103:753–765
Watanabe A, Nong VH, Zhang D, Arahira M, Yeboah NA, Udaka K, Fukazawa C (1999) Molecular cloning and ethylene-inducible expression of Chib1 chitinase from soybean (Glycine max (L.) Merr.). Biosci Biotechnol Biochem 63:251–256
Wei G, Kloepper JW, Tuzun S (1991) Induction of systemic resistance of cucumber to Colletotrichum orbiculare by select strains of plant growth-promoting rhizobacteria. Phytopathology 81:221–224
Weller DM, Cook RJ (1986) Increased growth of wheat by seed treatments with fluorescent pseudomonads, and implication of Pythiumi control. Can J Plant Pathol 8:328–334
Xie Z, Staehelin C, Wiemken A, Broughton W, Muller J, Boller T (1999) Symbiosis-stimulated chitinase isoenzymes of soybean (Glycine max (L.) Merr.). J Exp Bot 50:327–333
Xue L, Charest PM, Jabaji-Hare SH (1998) Systemic induction of peroxidases 1,3-β-glucanases chitinases and resistance in bean plants by binucleate Rhizoctonia species. Phytopathology 88:359–365
Yedidia I, Benhamou N, Kapulnink Y, Chet I (2000) Induction and accumulation of PR proteins activity during early stages of root colonization by the mycoparasite Trichoderma harzianum strain T-203. Plant Physiol Biochem 38: 863–873
Zhou T, Paulitz TC (1994) Induced resistance in the biological control of Pythium aphanidermatum by Pseudomonas spp. on European cucumber. J Phytopath 142:51–63
Acknowledgements
This work was supported by an NSERC grant to Dr. Donald L Smith.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jung, W.J., Mabood, F., Kim, T.H. et al. Induction of pathogenesis-related proteins during biocontrol of Rhizoctonia solani with Pseudomonas aureofaciens in soybean (Glycine max L. Merr.) plants. BioControl 52, 895–904 (2007). https://doi.org/10.1007/s10526-007-9089-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-007-9089-x