Abstract
As we age, there is an age-related loss in skeletal muscle mass and strength, known as sarcopenia. Sarcopenia results in a decrease in mobility and independence, as well as an increase in the risk of other morbidities and mortality. Sarcopenia is therefore a major socio-economical problem. The mechanisms behind sarcopenia are unclear and it is likely that it is a multifactorial condition with changes in numerous important mechanisms all contributing to the structural and functional deterioration. Here, we review the major proposed changes which occur in skeletal muscle during ageing and highlight evidence for changes in physical activity and nutrition as therapeutic approaches to combat age-related skeletal muscle wasting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Skeletal muscle ageing
Sarcopenia is defined as the loss of muscle mass and function as we age (Rosenberg 1989). In humans, sarcopenia affects individuals from approximately the 4th decade of life (Lexell et al. 1988), with a decrease of 30–50% in skeletal muscle mass and function by the time individuals reach approximately 80 years of age (Akima et al. 2001) and this is worsened by unloading of muscle in inactive old people (Bamman et al. 1998; Breen et al. 2013).
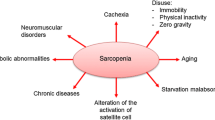
The mechanisms that underlie sarcopenia are not completely understood and it is likely that it is a multifactorial condition with a network of interacting dysfunctional systems (Fig. 1). Among several proposed processes are: decrease in protein synthesis (Welle et al. 1993), infiltration of fat tissue and connective tissue into skeletal muscle (Brack et al. 2007; Addison et al. 2014), dysregulation of proteasomal degradation pathways (Chondrogianni et al. 2000; Cuervo and Dice 2000), mitochondrial dysfunction (Short et al. 2005; Sakellariou et al. 2013), reduced number of satellite cells (Shefer et al. 2006), increased ROS production (Broome et al. 2006; Palomero et al. 2013) and increased inflammation (Fagiolo et al. 1993). These processes are proposed to lead to a decrease in muscle fibre number, decreased muscle cross-sectional area and defective regeneration observed in older humans (Lexell et al. 1988; Carlson et al. 2001). Fibre type changes have been proposed to be one of the important mechanisms of loss of muscle function with ageing, with type II fibres being more susceptible than type I fibres to atrophy (Larsson et al. 1978; Lexell et al. 1988; Nilwik et al. 2013). There is also evidence for increases in the ratio of type I to type II fibres in humans (Larsson et al. 1978; Larsson 1995; Andersen 2003; Lee et al. 2006). However, conflicting data exists demonstrating no difference in the percentage of type I and type II fibres with age in humans (Lexell et al. 1988). The reasons for these contradicting findings are unclear however it may be due to the age range of participants in Lexell’s study being slightly younger than the other studies. Furthermore, the demographics and lifestyles of the participants are not stated in the majority of the studies, therefore it is possible this may affect results. For example, the level of activity may have been lower in the younger individuals, and higher in the older participants than those in other studies in Lexell’s study.
Changes in satellite cells during sarcopenia
Satellite cells are stem cells present in adult muscle and are necessary for skeletal muscle to regenerate following injury (Shafiq and Gorycki 1965; Fry et al. 2015). During ageing, the number of satellite cells have been shown to decline in mice (Day et al. 2010; Chakkalakal et al. 2012) in a fibre type specific manner; in humans a decrease in satellite cells in type II fibres, with no differences seen in type I fibres have been shown in humans (Verdijk et al. 2014). Furthermore, a decline in the satellite cell content of extensor digitorum longus (EDL) muscle (primarily type II fibre) of mice is seen at 1 year of age, whereas this decline was not seen in the soleus muscle (predominately type I fibre) until mice were 2.5 years old (Shefer et al. 2006). Remarkably, this study also showed the presence of whole myofibres with no satellite cells present in 2.5 year old mice (Shefer et al. 2006). However, a study by Carlson et al. (2001) showed that satellite cell number was increased in muscles of old rats suffering from hind-limb neuropathy (Carlson et al. 2001). These studies used different species as well as different muscles and suggest that changes in satellite cell number may be specific to the muscle and species used. Ballak et al. showed that proteins involved in satellite cell proliferation from mice were not grossly affected during ageing (Ballak et al. 2015), however in a study by Shefer et al. the initial proliferation rate of isolated satellite cells from old mice in culture was lower than in cells isolated from younger mice (Shefer et al. 2006).
Satellite cells normally self-renew the quiescent pool of satellite cells (Zammit et al. 2004). During ageing the ability of satellite cells to self-renew is reduced (Shefer et al. 2006) due to the increase in proliferation (Chakkalakal et al. 2012) which can lead to apoptosis or senescence (Sousa-Victor et al. 2014). This may contribute to sarcopenia development and is associated with poor regeneration of muscle of aged animals (Carlson et al. 2001). Furthermore, the loss of satellite cells has been associated with neuromuscular degeneration during ageing (Liu et al. 2017).
The role of altered properties of satellite cells as an underlying cause of sarcopenia is unclear, as depletion of satellite cells from muscles of old mice had no effect on the cross-sectional area of the muscle (Fry et al. 2015) or on muscle growth after unloading (Jackson et al. 2012). Moreover, parabiosis studies showed that muscle of old mice can regenerate successfully when placed in a young host (Carlson and Faulkner 1989; Conboy et al. 2005). In vitro studies have also shown that satellite cells isolated from old mice can differentiate into mature myotubes (Shefer et al. 2006) and when supplemented with fibroblast growth factor (FGF), no difference was shown in the ability of satellite cells from adult and old mice to proliferate ex vivo. These data suggest that the changes in the satellite cell environment, rather than loss of function within satellite cells, during ageing are likely to cause the dysfunction of satellite cells (Shefer et al. 2006; Lee et al. 2013). However, the ablation of satellite cells results in increased fibrosis suggesting satellite cell function may play a role in preventing fibrosis. Further studies have also shown that satellite cells are essential for regeneration following damage in muscle since ablation of these cells had profound effects on the ability of muscles to successfully regenerate in mice (Fry et al. 2015).
Some of the major pathways associated with these changes in satellite cells during ageing include Notch and Wnt signalling. Notch signalling is associated with the proliferation of satellite cells whereas canonical Wnt signalling is associated with the differentiation of muscle cells (Brack et al. 2007), however the involvement of Wnt in muscle differentiation is debated (Murphy Malea et al. 2014). During ageing there is a decrease in Notch signalling (Carey et al. 2007) and a switch from the canonical Wnt signalling to non-canonical Wnt signalling, this results in the prevention of the self-renewal ability of satellite cells that is seen in the aged satellite cell (Florian et al. 2013). Key genes which regulate skeletal muscle development, MyoD and Myf5 are also increased in the aged muscle in humans, mouse and rats (Hameed et al. 2003; Raue et al. 2006; Chakkalakal et al. 2012).
Changes in protein synthesis during sarcopenia
A balance between protein synthesis and degradation is vital to maintain muscle mass and the relevant gains or losses in protein synthesis and degradation rates are required for hypertrophy and atrophy. Studies of basal levels of protein synthesis have shown contradicting results with some studies demonstrating decreased rate of overall protein synthesis in the muscle of old compared with adult humans (Hasten et al. 2000) and other studies showing no difference in protein synthesis in muscles of old humans compared with muscles of adults (Volpi et al. 2001; Wall et al. 2015; Francaux et al. 2016). Thus, there is a lack of consistent evidence for differences in basal protein synthesis between young and old people. Therefore, research has focused on studying post-prandial state protein synthesis to identify whether older people can utilise protein as efficiently as younger people. These studies have shown that older people have a blunted protein synthesis response to nutrients (Cuthbertson et al. 2005; Wall et al. 2015) and to exercise (Fry et al. 2011); this is known as anabolic resistance. Data by Koopman et al. (2009) demonstrated that there was no difference in either digestion or absorption of proteins between the old and young people therefore anabolic resistance was proposed to result from an increase in the amount of protein required to reach a ‘threshold’ for protein synthesis to occur (Koopman et al. 2009). This is further evidenced by studies that show blunted mTOR activation following protein intake in the muscle of older people (Cuthbertson et al. 2005). Although anabolic resistance is likely to contribute to the onset of sarcopenia, it is unlikely that it contributes to the continuous decrement in muscle mass seen in sarcopenia as an increase in anabolic resistance did not have any detrimental effect on muscle mass (Smeuninx et al. 2017).
Changes in protein degradation during sarcopenia
The appropriate quality control of protein is vital for the correct functioning of the cell. Two common mechanisms responsible for this are the proteasomal degradation pathway and autophagy. These two pathways are dysregulated in a host of tissues during ageing and therefore are hypothesised to contribute to the loss of muscle mass with age.
Proteasomal degradation during sarcopenia
The role of the ubiquitin–proteasome system (UPS) is to regulate protein degradation and maintain protein homeostasis. Proteins are labelled with ubiquitin molecules for degradation and are passed to the proteasome where they are degraded.
There are numerous ligases able to carry out protein degradation; however Atrogin-1 and muscle RING-finger protein-1 (Murf1) are muscle specific ligases that play a role in numerous models of muscle atrophy (Bodine et al. 2001). Despite the evidence for a role of the UPS in muscle atrophy, the role of the UPS in sarcopenia is controversial. Some studies have shown the upregulation of both Atrogin-1 and Murf1 levels in the muscle of old rats (Clavel et al. 2006), whilst others have shown no difference or downregulation between the age groups (Gaugler et al. 2011) or the upregulation of only one of the atrogenes (Altun et al. 2010). The contrasting results in these studies maybe due to the transient nature of these two atrogenes making it difficult to accurately identity changes in their expression levels (Bodine et al. 2001; Sacheck et al. 2007).
Autophagy during sarcopenia
Autophagy is the process of “self-eating” and is crucial for the turnover of cell components, both in normal circumstances as well as during cellular stress such as starvation (Pfeifer and Warmuth-Metz 1983). As opposed to the UPS which is only able to degrade proteins, the lysosomal system is able to incorporate protein aggregates, macromolecules and whole organelles (Korovila et al. 2017). Reduced autophagy has been seen in many cell types and tissues during ageing (Cuervo and Dice 2000; Kiffin et al. 2007) and there is evidence that autophagy is dysregulated in the muscle of old rodents (Russ et al. 2012; Joseph et al. 2013b; Russ et al. 2015a). Studies in Drosophila have shown accumulation of protein aggregates in muscle that was associated with impaired muscle function (Demontis and Perrimon 2010), providing evidence for autophagic dysregulation in the development of sarcopenia.
Impairment of mitophagy (autophagy of the mitochondria) is detrimental to muscle homeostasis, and leads to the accumulation of damaged and dysfunctional mitochondria (Grumati et al. 2010). Dysfunctional mitophagy has been shown to occur in the muscle of old men (Gouspillou et al. 2014) and women (Drummond et al. 2014) and is therefore hypothesised to play a role in the mitochondrial dysfunction seen in sarcopenia.
Infiltration of fat and fibrosis during sarcopenia
Fibrosis is the accumulation of extracellular matrix (Alnaqeeb et al. 1984; Goldspink et al. 1994) and during sarcopenia both fibrosis and the infiltration of fat into skeletal muscle occurs (Evans et al. 1995; Song et al. 2004). This decrease in the quality of skeletal muscle is thought to contribute to the age-related impairment in force generation, particularly in lateral transfer of force throughout the muscle fibres (Ramaswamy et al. 2011).
The accumulation of extracellular matrix particularly collagen, seems to be the result of incomplete repair of muscle following damage (Serrano and Munoz-Canoves 2010). Skeletal muscle regeneration following injury depends upon a series of well-co-ordinated events involving numerous cell types that modify the microenvironment of the damaged muscle which is essential for normal muscle regeneration to preserve muscle architecture. During ageing, this remodelling becomes dysregulated. Dysfunction in remodelling is coupled with a switch in myogenic progenitor cells from a myogenic to fibrotic fate (Shefer et al. 2006; Brack et al. 2007) or an adipogenic fate (Vettor et al. 2009; Pisani et al. 2010) suggesting satellite cells as a possible source of intramuscular fibrotic and fat deposition. This change in cell fate is possibly due to changes in the Wnt signalling pathway which has been shown to be involved in the myogenic fate of satellite cells and increased Wnt signalling has been shown to occur in ageing muscle (Vertino et al. 2005; Brack et al. 2007). Alternatively, changes in the inflammatory responses, such as those seen during ageing, may also play a role in determining cell fate (Wang et al. 2015).
Increases in collagen deposition lead to an increase in advanced glycation end products (AGE) in skeletal muscle in humans (Haus et al. 2007) and changes in total muscle collagen and the endomysium and perimysium have been shown to correlate with the increase in stiffness of muscle and a decline in muscle tension with age (Alnaqeeb et al. 1984). However, Goldspink et al. showed no difference in the transcription levels of collagen in the muscle of old mice (Goldspink et al. 1994). Given that total collagen levels are elevated in muscle of old mice these data suggest that there may be a reduction in collagen degradation possibly due to the increased collagen crosslinking, making the collagen somewhat resistant to degradation by collagenase.
Changes in the neuromuscular system during sarcopenia
During ageing, a decrease in motor unit number in various muscles of humans (Piasecki et al. 2015) rodents (Ling et al. 2009; Sheth et al. 2018). A decrease in the number of motor axons innervating fibres has been observed in rodents (Ansved and Larsson 1990) and humans (Tomlinson and Irving 1977). Denervation leads to the sprouting of axons of existing functional nerves to innervate fibres in close proximity. This is known as motor unit remodelling and is evidenced by an increase in reinnervation is old mice (Larsson 1995). Reinnervation is speculated to cause some of the age-related fibre- type switching that occurs (Larsson et al. 1978; Andersen 2003; Lee et al. 2006), as slow motor neurons may be more adapted to reinnervation which leads to an age-related loss in fast motor neurons (Kadhiresan et al. 1996). If reinnervation does not occur, it is likely that the muscle fibre will eventually undergo cell death (Borisov and Carlson 2000; Borisov et al. 2001; Vasilaki et al. 2016). Research has provided evidence that neuromuscular remodelling is a pre-requisite for muscle atrophy (Deschenes et al. 2010; Sheth et al. 2018). Sheth et al. have shown that the decrease in motor unit number occurred before the loss of muscle function and the loss of motor unit connectivity seen during ageing correlates with muscle size and contractibility (Sheth et al. 2018). Deschenes et al. also showed that denervation precedes muscle loss in rats during ageing (Deschenes et al. 2010). However this is still poorly understood, due to the confines to study the neuromuscular system during ageing, one of these limitations is that it is not always possible to use human nerve and muscle tissues and these processes are only able to be studied using in vivo animal models or ex vivo co-cultures that require the use of spinal cord explants from animal embryos as a source of motor neurons. However, new developments in techniques to derive functional motor neurons from human pluripotent stems cells now allow development of alternative approaches.
Increases in reactive oxygen species and alterations in antioxidant defence systems during sarcopenia
Reactive oxygen species (ROS) are extremely reactive molecules and have important roles in metabolism and cell signalling (Thannickal and Fanburg 2000). Though ROS have important functions in cells, when in excess if not eliminated by the antioxidant defence system, ROS can be damaging to cellular marcomolecules such as lipids, proteins and DNA, leading to cell death.
ROS are increased in the satellite cells of older subjects (Minet and Gaster 2012) which may contribute to the loss of regeneration potential in muscles of older animals and humans. The basal levels of ROS are also increased in mouse muscle during ageing (Palomero et al. 2013). This increase in ROS is thought to be detrimental to skeletal muscle as it is reflected by increases in markers of oxidative damage such as an increase in protein carbonyl and malonaldehyde and oxidation of lipids, DNA and proteins in the muscles of old mice (Mecocci et al. 1999; Broome et al. 2006; Sakellariou et al. 2016). This modified redox status has also been shown to be detrimental for other processes such as calcium transport (Fulle et al. 2003) and increased degradation of important proteins such as myogenic proteins, impaired autophagy (Scherz-Shouval et al. 2007) and inhibition of differentiation of muscle cells (Ardite et al. 2004; Sandiford et al. 2014).
ROS are eliminated by the antioxidant defence system. During ageing, it has been shown there is a constituent upregulation of the antioxidant defence system activity in skeletal muscle (Vasilaki et al. 2006; Palomero et al. 2013; Sullivan-Gunn and Lewandowski 2013). Following a stress such as muscle contraction, there is no further increase in antioxidant defence enzyme activities in the muscle of old humans and animals (Vasilaki et al. 2006; Ryan et al. 2008) potentially leaving the cells exposed to oxidative damage.
The contribution that ROS play in the muscle ageing process remains unclear. Interestingly, overexpression of copper/zinc (Cu/Zn) superoxide dismutase (CuZnSOD) leads to muscle atrophy (Rando et al. 1998) whereas the deletion of CuZnSOD results in the inability of muscle to adapt to stress and decreased muscle force generation in mice (Muller et al. 2006; Vasilaki et al. 2010; Larkin et al. 2011; Sakellariou et al. 2014b) suggesting that the redox balance is an important modulator of sarcopenia.
Dysfunction of mitochondria during sarcopenia
Mitochondria are essential for providing the ATP required for muscle contraction and are also central to the redox regulation and quality control of the cell and therefore for the viability of muscle cells. Given this essential role of mitochondria in skeletal muscle maintenance and survival, alterations in mitochondria are considered one of the primary contributors driving the sarcopenic process.
The role of the mitochondria in sarcopenia was proposed by Miquel et al. in the mitochondrial free radical theory of ageing (Miquel et al. 1980). This stated that mitochondrial dysfunction in ageing occurs from the increase in ROS and blunted antioxidant defences; these damaging effects change the redox status of the cell which in turn leads to mutations in the mitochondrial DNA (mtDNA) leading to the production of dysfunctional components of the electron transport chain (ETC). Impairment of the ETC leads to compromised oxidative phosphorylation which causes a further rise in ROS, causing a vicious circle which exacerbates the ageing phenotype (Miquel et al. 1980).
This hypothesis was confirmed in skeletal muscle by studies showing that during ageing there was an increase in ROS, mtDNA deletions and mitochondrial dysfunction which were associated with skeletal muscle atrophy in non-human primates (Lee et al. 1998a) rodents (Wanagat et al. 2001) and humans (Bua et al. 2006). Interestingly, these observations were not seen in the phenotypical normal regions of the muscle fibres. Furthermore mice which contain error prone mtDNA polymerase accumulate high levels of mtDNA mutations and show severe muscle atrophy due to increased apoptosis (Kujoth et al. 2005). Further studies showed that mtDNA mutations lead to increased ROS production (Logan et al. 2014) and overexpression of antioxidants have been shown to protect against some of the oxidative damage as well as changes to mitochondrial respiration and ATP production in skeletal muscle (Lee et al. 2010) which prevent age related mitochondrial dysfunction. These data suggest that both ROS and mitochondrial dysfunction are likely to contribute to sarcopenia. However, more recently the mitochondrial free radical theory of ageing has become debatable as non-mitochondrial sources of ROS generation have been identified (Sakellariou et al. 2013, 2014a; Jackson and McArdle 2016).
Mitochondria in the muscle of sarcopenic individuals also show increased fusion and decreased fission (Yoon et al. 2006) and impairment of mitochondrial autophagic (Gouspillou et al. 2014) and proteasomal machinery (Marzetti et al. 2008). The release of damaged mitochondrial components into the extracellular matrix correlates with increases in pro-inflammatory cytokines in the plasma of elderly humans (Pinti et al. 2014). Mitochondria undergo complex morphological changes during aging which are also likely to affect their function (Leduc-Gaudet et al. 2015) and thus give further evidence for a role for dysfunctional mitochondria in sarcopenia.
Increased inflammation during sarcopenia
The inflammatory response is the secretion of pro-inflammatory mediators in response to the appropriate stimuli such as toxins, bacteria, foreign bodies or infection and restores homeostasis and initiates repair. The acute pro-inflammatory state is vital for the repair of cells but too much for too long is thought to be detrimental; for example chronic low grade inflammation has been associated with ageing and has been implicated in numerous conditions and diseases (Lagrand et al. 1999; Duncan et al. 2003; Frischer et al. 2009).
Low level chronic inflammation coupled with immunosenescence, the decline in the function of the immune system with age, that occurs in ageing has been termed ‘inflamm-ageing’ (Franceschi et al. 2000). Inflamm-ageing has been associated with numerous age-related diseases and conditions (Chung et al. 2009) and has been implicated as a major contributor to sarcopenia (Schaap et al. 2006, 2009).
Serum levels of TNF-α, IL6 and C-reactive protein (CRP) are all increased in ageing and have been proposed to be important mediators of sarcopenia as changes are correlated with a decrease in muscle mass (Pedersen et al. 2003; Aleman et al. 2011; Bian et al. 2017), performance (Thalacker-Mercer et al. 2010), function (Bautmans et al. 2011), strength (Tiainen et al. 2010; Norman et al. 2014) and fitness (Levinger et al. 2010). As well as increasing myokine production (Lightfoot et al. 2015). It should be noted however that TNF-α, IL6 and CRP have all been shown to have beneficial effects in skeletal muscle growth; IL6 and TNF-α at low levels has been shown to cause satellite cell proliferation and differentiation (Li 2003; Kurosaka and Machida 2013), therefore it is likely that the effect of systemic inflammation on muscle mass and function during ageing may only occur when it surpasses a certain threshold and/or persists for an extended period (Degens 2010).
The increase in inflammation leads to a further increase in ROS production by skeletal muscle (Li et al. 1998). As well as an increase in skeletal muscle cell apoptosis (Phillips and Leeuwenburgh 2005), inflammation has also been proposed to play a role in the anabolic resistance described previously and high levels of inflammation have been associated with catabolism of skeletal muscle (Li et al. 1998; Cuthbertson et al. 2005).
Current therapies for sarcopenia
The ageing population is growing substantially with 617 million people worldwide currently 65 and over and demographic analysis predict that this will increase to 1.6 billion by 2050 (He et al. 2016). Although there have been improvements in lifespan, the same advances have not been made in health-span, meaning that the extra years of living are under poor health. Sarcopenia is a major contributor to frailty in the older population resulting in further immobility with a loss of independence, as well as increasing the risk of other chronic diseases and morbidity (Coin et al. 2013). This immobility and co-morbidities can be further escalated through lifestyle choices such as a sedentary diet and a poor diet. Thus sarcopenia has major socio-economic costs—in 2000 the US spent 1.5% of their national budget ($18.5 billion) on sarcopenia (Janssen et al. 2004), signifying the importance of finding a treatment or preventative therapy for sarcopenia. This section focuses on modifiable lifestyle factors which are economically resourceful compared with drug interventions.
Physical exercise and exercise
The importance of physical activity in preventing sarcopenia has been shown in studies where people who are less physically active have a higher chance of developing sarcopenia (Lee et al. 2007). It is generally thought that exercise and physical activity are beneficial and can attenuate some of the detrimental effects of unloading and bed rest on muscle loss in adult and old individuals (Caiozzo et al. 2009; Belavy et al. 2014; McMahon et al. 2014; Valenzuela et al. 2018). It is important to define the differences between exercise and physical activity. Physical activity is defined as bodily movements that are produced by skeletal muscles and result in energy expenditure; examples of this include walking and house chores. Exercise is a subset of physical activity that is planned, structured, and repetitive and has as a final objective to improve or maintain physical fitness (Caspersen et al. 1985). Lifelong exercise was shown to be associated with modest improvements in muscle mass in the quadriceps of mice (McMahon et al. 2014) and lifelong triathlon training is able to preserve muscle mass in the mid-thigh of humans (Wroblewski et al. 2011). However, the benefits of exercise on muscle function are controversial since lifelong exercise did not prevent the loss of strength when it was shown that master athletes still undergo a loss in strength, power and endurance with age (Grassi et al. 1991; Kayani et al. 2008). Others have also shown no correlation between physical activity and the maintenance of muscle mass (Mitchell et al. 2003) and only a higher level of physical activity and not ‘leisure-time’ activity are able to prevent or delay some sarcopenic effects (Raguso et al. 2006).
There are various different exercise programmes that are recommended for the older population in an attempt to combat sarcopenia.
Resistance training
Resistance training is the requirement to generate force to move or resist weight such as weight lifting/push ups/leg press. Numerous studies have shown beneficial effects of resistance training in the function of skeletal muscle of older people; increasing muscle mass and strength (Fiatarone et al. 1994; Maltais et al. 2015; Tsuzuku et al. 2018) as well as cross-sectional area of myofibres (Fiatarone et al. 1994; Leenders et al. 2013; Ribeiro et al. 2017) and motility (Fiatarone et al. 1994; Liu and Latham 2009).
Improvements in muscle function following resistance training are thought to be due to an improved neuromuscular system (Taaffe et al. 1999), increased protein synthesis and attenuation of anabolic resistance (Schulte and Yarasheski 2001) and this is associated with an increase in the satellite cell content of type II fibres (Verdijk et al. 2009a; Leenders et al. 2013). Resistance training has also been linked with a decrease in catabolic and increase in anabolic pathways (Ribeiro et al. 2017).
Aerobic training
Aerobic training stimulates the heart and blood flow and provides cardiovascular conditioning such as running, cycling and swimming. Aerobic exercise has been shown to result in increased cross-sectional area of muscle fibres and hypertrophy of muscles of older humans (Schwartz et al. 1991; Konopka et al. 2013). However, the effects of aerobic exercise are not as well established as resistance exercise and it is likely that the hypertrophic effects of aerobic exercise depend on the frequency, intensity and length of exercise.
The effects of aerobic exercise on skeletal muscle are primarily through increases in mitochondrial proteins such as cytochrome C and PGC-1α (Short et al. 2003; Konopka et al. 2013). Increases in mitochondrial biogenesis result in improved mitochondrial function, metabolic control and respiratory capacity (Coggan et al. 1992; Short et al. 2003) consequently increasing the endurance of the individual. Furthermore, long term aerobic exercise programmes have shown an ability to reduce ROS production by muscle in old people (Ghosh et al. 2011). Aerobic exercise has also been shown to decrease anabolic resistance through the upregulation of protein synthesis through the Akt/mTOR pathway (Fujita et al. 2007), as well decreasing inflammation (Kohut et al. 2006).
Other forms of physical activity
Other forms of physical activity include power training. Power declines at a rate of 3–4% per year in older people and this is detrimental for everyday activities such as climbing stairs. To improve power, fast shortening resistance training is implemented. Improvements in skeletal muscle power (Fielding et al. 2002; Henwood and Taaffe 2005; Reid et al. 2008) and in the ability to carry out every day activities in older people have been seen following power exercise regimes (Henwood and Taaffe 2005). These improvements are thought to be due to changes in the neuromuscular junction that allow better recruitment of the motor units and therefore an increase in the firing rate of fast twitch fibres (Fielding et al. 2002; Reid et al. 2008). Reid et al. have shown power training to be more effective than slow velocity resistance training (Reid et al. 2008).
Many suggested physical activities may be too intense for older adults to maintain over a prolonged time. To combat this, less impact exercises such as whole-body vibrations and whole body electro-myostimulation have been developed. These techniques use impulses that cause involuntary contractions of the muscles to preferentially recruit the fast twitch fibres that are most affected by ageing and this approach has been shown to increase maximum isometric strength and muscle mass (Kemmler et al. 2010, 2014) and grip strength in older women (Stengel et al. 2015).
Protein intake and calorie restriction
Protein and other nutrients are vital for the protein synthesis required for muscle growth and maintenance. Therefore it is proposed that nutritional intake may play a role in sarcopenia and altering nutritional intake may be able to relieve some symptoms of sarcopenia.
Increase in protein intake
In addition to the anabolic resistance that occurs with age, around 30–40% of women and 20–40% of men over 50 do not reach the recommended daily intake of protein and it is has been shown that a low protein diet can be detrimental to muscle (Oumi et al. 2000; Balasa et al. 2011; Tarry-Adkins et al. 2016). Therefore, a considerable number of studies examining interventions against sarcopenia have focused on increasing protein intake.
Studies have shown that increasing the overall amount of protein intake can at least overcome the anabolic resistance in older people leading to an increase in protein synthesis, muscle mass and decreased proteolysis in rodents (Mosoni et al. 2014) and humans (Genaro et al. 2015; Moore et al. 2015; Norton et al. 2015; Verreijen et al. 2015).
The importance of the essential amino acid profile, digestibility and bioavailability of ingested protein on the anabolic potential of protein was demonstrated in studies where anabolic resistance was overcome by increasing the percentage of leucine or essential amino acids contained in the ingested protein rather than the total amount of protein (Volpi et al. 2003). Increased protein synthesis was also achieved by inhibiting co-ingestion of carbohydrates and protein (Katsanos et al. 2006). This improvement in anabolic resistance in the old is likely to be through the upregulation of the Akt/mTOR pathway as well as decreasing proteolysis and autophagy (Volpi et al. 2003). Increases in Akt/mTOR pathway and decreases in proteolysis have also been shown in vitro (Sato et al. 2014) and in vivo resulting in increased muscle mass when used as a single supplement of leucine or in combination with other nutrients (Sato et al. 2013, 2015). Leucine supplementation led to improved muscle regeneration in old rats through a decrease in inflammation and an increase in satellite cell proliferation resulting in an increase in the cross-sectional area of regenerated fibres compared with control animals (Pereira et al. 2015).
In contrast, meta-analysis of protein supplementation studies (Xu et al. 2014) showed no difference between the effect of protein supplementation and that of a placebo groups on muscle mass, protein synthesis and muscle strength in older men (Dirks et al. 2014) or women (Zhu et al. 2015). Furthermore, (Russ et al. 2015a, b) showed that protein supplementation attenuated muscle degradation through decreasing Murf1 expression, this did not translate to any functional benefits to muscles of old rats (Russ et al. 2015b). However these discrepancies may be explained by the times at which intake of protein occurred; Symons et al. (2007) showed that a 90 g of protein meal in humans does not cause more protein synthesis than a 30 g meal in humans (Symons et al. 2007). This suggests that ingestion of more than 30 g of protein in one meal is an energetically inefficient means of protein synthesis and that protein intake should be spread out throughout the day to optimise muscle protein synthesis.
Despite the benefits of increased protein synthesis on sarcopenia, it is important to note that high protein diets (3 g protein × kg fat-free mass (FFM)(− 1) × day(− 1) have been linked to a decrease in the glomerular filtration rate in older people, suggesting that high levels of protein may have damaging effects on the kidney (Walrand et al. 2008) and undesirable effects on the musculoskeletal system were seen when high protein diets led to negative a calcium balance that could lead to osteoporosis in men (Allen et al. 1979). Thus prescribing increased intake of protein in old people is controversial.
Calorie restriction
Calorie restriction is thought to be one of the most effective interventions of attenuating ageing. Restriction of the number of calories eaten has been proved to be life-extending in numerous species (Weindruch et al. 1986; Lakowski and Hekimi 1989; Jiang et al. 2000) as well as reducing all-cause mortality in rhesus monkeys (Colman et al. 2014).
The benefits of calorie restriction have been extended into sarcopenia. In rats, a 6 week 20% reduction in calorie intake led to an attenuation of age-related loss of muscle mass and function in the soleus and gastrocnemius muscles through an upregulation of PGC-1α (Joseph et al. 2013a). Calorie restriction also preserved fibre number and type and protected mitochondrial DNA from deletion (Lee et al. 1998b). In rats, calorie restriction decreased apoptosis and protected from oxidative stress (Dirks and Leeuwenburgh 2004) as well as a decrease in the overall oxidation status in skeletal muscle (Hepple et al. 2008). These data suggest that calorie restriction prevents sarcopenia potentially through an inhibition of apoptosis and enhancement of the mitochondrial function and this has been shown to occur through the upregulation of the NAD-deacetylase Sirt1 (Cohen et al. 2004). Sarcopenia was also attenuated by calorie restriction in the rhesus monkey (Colman et al. 2008).
The relevance and beneficial effects of human calorie restriction is shown in studies which have shown positive effects in diseases such as diabetes and atherosclerosis (Fontana et al. 2004; Weiss et al. 2006). Importantly Mercken et al. showed a long term 30% reduction in calorie intake in humans changed the transcriptional profile in skeletal muscle of an older individual similar to that of a younger subject, increased the production of antioxidants and decreased inflammation (Mercken et al. 2013). This suggests that the benefits of calorie restriction can be extended into human muscle however a lot more work is needed in this area. It is likely that for a high adherence and for beneficial effects of a calorie restricted diet, this would have to be implemented at a younger age and it would be vital for people to be well informed about calorie intake. This would also need to be looked at on an individual basis as insufficient nutrition is already a problem for a lot of elderly people therefore, if misinformed it could lead to the malnutrition of patients which has been shown to result in a lower muscle mass (Pierik et al. 2017).
Protein supplementation paired with exercise
Given the benefit of exercise and protein intake on sarcopenia, numerous studies have shown that combined together, protein and exercise can increase muscle strength and mass in the old (Tieland et al. 2012; Shahar et al. 2013; Maltais et al. 2015; Palop et al. 2015).
The ability of protein supplementation to increase muscle mass and strength further than with exercise alone is debateable. Some research groups have suggested that protein supplementation will only enhance exercise-induced muscular improvements if there is an existing protein deficiency (Verdijk et al. 2009b). This is particularly relevant in those who do not already reach the recommended daily intake of protein, where the amount and distribution of protein throughout the day alongside an effective exercise plan may play an essential part in whether the supplement will be effective.
Future direction
Despite some evidence for the benefits of exercise and nutritional interventions on sarcopenia there is still no intervention that there is an agreement that is beneficial to sarcopenia. Studies looking at more pharmacological agents have been more promising. For example the inhibition of myostatin, a negative regulator of muscle mass, increased muscle size in mice and cattle (Lee 2007) and the use of sex hormones have improved muscle strength and mass (Stárka 2006). More recently microRNAs, small RNAs that post transcriptionally regulate gene expression, have been shown to be involved in skeletal muscle development (Goljanek-Whysall et al. 2012) and the levels of miRNAs dysregulated in ageing humans (Drummond et al. 2011) and mice (Soriano-Arroquia et al. 2016a, b). Furthermore the restoration of level of miRNAs have led to an improved muscle phenotype in old mice, but also the imitation of ageing miRNA levels in younger mice have also resulted in detrimental effects in the muscle (Soriano-Arroquia et al. 2016a). Furthermore, miRNAs have also been shown to be involved in the adaption of skeletal muscle following exercise (Russell et al. 2013) thus showing the potential for miRNAs paired with a personalised exercise regime as a treatment for sarcopenia.
Conclusion
Skeletal muscle is a vital organ to the body and the age-related changes that occur in the muscle are detrimental to the correct functioning of skeletal muscle and leads to the loss of independence. The ever-increasing ageing population is an important socio-economic problem. The changes that occur in sarcopenia have been described in the sections above and there is a vast amount of evidence that these changes contribute to the resulting phenotype in skeletal muscle wasting.
Despite the huge amount of work looking at life-style changes on sarcopenia, whether these changes can prevent or cure sarcopenia is still to be established. The contrast in the results from these studies suggests that the responsiveness of individuals to exercise and changes in nutritional intake may depend on the individual and stage of sarcopenia that is occurring. It may suggest that complete personalised regimes, maybe in conjunction with pharmacological interventions are required for full function of the muscle during later life.
References
Addison O, Drummond MJ, LaStayo PC, Dibble LE, Wende AR, McClain DA et al (2014) Intramuscular fat and inflammation differ in older adults: the impact of frailty and inactivity. J Nutr Health Aging 18:532–538
Akima H, Kano Y, Enomoto Y, Ishizu M, Okada M, Oishi Y et al (2001) Muscle function in 164 men and women aged 20–84 yr. Med Sci Sports Exerc 33:220–226
Aleman H, Esparza J, Ramirez FA, Astiazaran H, Payette H (2011) Longitudinal evidence on the association between interleukin-6 and C-reactive protein with the loss of total appendicular skeletal muscle in free-living older men and women. Age Ageing 40:469–475
Allen LH, Oddoye EA, Margen S (1979) Protein-induced hypercalciuria: a longer term study. Am J Clin Nutr 32:741–749
Alnaqeeb MA, Al Zaid NS, Goldspink G (1984) Connective tissue changes and physical properties of developing and ageing skeletal muscle. J Anat 139:677–689
Altun M, Besche H, Overkleeft H, Piccirillo R, Edelmann MJ, Kessler BM et al (2010) Muscle wasting in aged, sarcopenic rats Is associated with enhanced activity of the ubiquitin proteasome pathway. J Biol Chem 285:39597–39608
Andersen J (2003) Muscle fibre type adaptation in the elderly human muscle. Med Sci Sports 13:40–47
Ansved T, Larsson L (1990) Quantitative and qualitative morphological properties of the soleus motor nerve and the L5 ventral root in young and old rats. Relation to the number of soleus muscle fibers. J Neurol Sci 96:269–282
Ardite E, Albert Barbera J, Roca J, Fernández-Checa JC (2004) Glutathione depletion impairs myogenic differentiation of murine skeletal muscle C2C12 cells through sustained NF-κB activation. Am J Pathol 165:719–728
Balasa A, Sanchez-Valle A, Sadikovic B, Sangi-Haghpeykar H, Bravo J, Chen L et al (2011) Chronic maternal protein deprivation in mice is associated with overexpression of the cohesin-mediator complex in liver of their offspring. J Nutr 141:2106–2112
Ballak SB, Jaspers RT, Deldicque L, Chalil S, Peters EL, de Haan A et al (2015) Blunted hypertrophic response in old mouse muscle is associated with a lower satellite cell density and is not alleviated by resveratrol. Exp Gerontol 62:23–31
Bamman MM, Clarke MSF, Feeback DL, Talmadge RJ, Stevens BR, Lieberman SA et al (1998) Impact of resistance exercise during bed rest on skeletal muscle sarcopenia and myosin isoform distribution. J Appl Physiol 84:157–163
Bautmans I, Onyema O, Van Puyvelde K, Pleck S, Mets T (2011) Grip work estimation during sustained maximal contraction: validity and relationship with dependency and inflammation in elderly persons. J Nutr Health Aging 15:731–736
Belavy D, Möhlig M, Pfeiffer A, Felsenberg D, Armbrecht G (2014) Exercise, strict physical inactivity (experimental bed-rest) and their effects on visceral adipose tissue and fat distribution. Obes Res Clin Pract 8:6–7
Bian A-L, Hu H-Y, Rong Y-D, Wang J, Wang J-X, Zhou X-Z (2017) A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-α. Eur J Med Res 22:25
Bodine S, Latres E, Baumhueter S, Lai V, Nunez L, Clarke B et al (2001) Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294:1704–1708
Borisov AB, Carlson BM (2000) Cell death in denervated skeletal muscle is distinct from classical apoptosis. Anat Rec 258:305–318
Borisov AB, Dedkov EI, Carlson BM (2001) Interrelations of myogenic response, progressive atrophy of muscle fibers, and cell death in denervated skeletal muscle. Anat Rec 264:203–218
Brack A, Conboy M, Roy S, Lee M, Kuo C, Keller C et al (2007) Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317:807–810
Breen L, Stokes KA, Churchward-Venne TA, Moore DR, Baker SK, Smith K et al (2013) Two weeks of reduced activity decreases leg lean mass and induces “anabolic resistance” of myofibrillar protein synthesis in healthy elderly. J Clin Endocrinol Metab 98:2604–2612
Broome CS, Kayani AC, Palomero J, Dillmann WH, Mestril R, Jackson MJ et al (2006) Effect of lifelong overexpression of HSP70 in skeletal muscle on age-related oxidative stress and adaptation after nondamaging contractile activity. FASEB J 20:1549–1551
Bua E, Johnson J, Herbst A, Delong B, McKenzie D, Salamat S et al (2006) Mitochondrial DNA-deletion mutations accumulate intracellularly to detrimental levels in aged human skeletal muscle fibers. Am J Hum Genet 79:469–480
Caiozzo VJ, Haddad F, Lee S, Baker M, Paloski W, Baldwin KM (2009) Artificial gravity as a countermeasure to microgravity: a pilot study examining the effects on knee extensor and plantar flexor muscle groups. J Appl Physiol 1985(107):39–46
Carey KA, Farnfield MM, Tarquinio SD, Cameron-Smith D (2007) Impaired expression of notch signaling genes in aged human skeletal muscle. J Gerontol Ser A 62:9–17
Carlson B, Faulkner J (1989) Muscle transplantation between young and old rats: age of host determines recovery. Am J Physiol 256:1262–1266
Carlson BM, Dedkov EI, Borisov AB, Faulkner JA (2001) Skeletal muscle regeneration in very old rats. J Gerontol Ser A 56:B224–B233
Caspersen CJ, Powell KE, Christenson GM (1985) Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep 100:126–131
Chakkalakal J, Jones K, Basson A, Brack A (2012) The aged niche disrupts muscle stem cell quiescence. Nature 490:355–360
Chondrogianni N, Petropoulos I, Franceschi C, Friguet B, Gonos E (2000) Fibroblast cultures from healthy centenarians have an active proteasome. Biol Ageing 35:721–728
Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY et al (2009) Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev 8:18–30
Clavel S, Coldefy AS, Kurkdjian E, Salles J, Margaritis I, Derijard B (2006) Atrophy-related ubiquitin ligases, atrogin-1 and MuRF1 are up-regulated in aged rat Tibialis Anterior muscle. Mech Ageing Dev 127:794–801
Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM et al (1992) Skeletal muscle adaptations to endurance training in 60- to 70-yr-old men and women. J Appl Physiol 72:1780–1786
Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B et al (2004) Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305:390–392
Coin A, Sarti S, Ruggiero E, Giannini S, Pedrazzoni M, Minisola S et al (2013) Prevalence of sarcopenia based on different diagnostic criteria using DEXA and appendicular skeletal muscle mass reference values in an italian population aged 20 to 80. J Am Med Dir Assoc 14:507–512
Colman RJ, Beasley TM, Allison DB, Weindruch R (2008) Attenuation of sarcopenia by dietary restriction in rhesus monkeys. J Gerontol Ser A 63:556–559
Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM (2014) Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun 5:3557
Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA (2005) Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433:760–764
Cuervo A, Dice J (2000) Age-related decline in chaperone-mediated autophagy. J Biol Chem 275:31505–31513
Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P et al (2005) Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 19:422–424
Day K, Shefer G, Shearer A, Yablonka-Reuveni Z (2010) The depletion of skeletal muscle satellite cells with age is concomitant with reduced capacity of single progenitors to produce reserve progenitors to produce reserve progeny. Dev Biol 340:330–343
Degens H (2010) The role of systemic inflammation in age-related muscle weakness and wasting. Scand J Med Sci Sports 20:28–38
Demontis F, Perrimon N (2010) FOXO/4E-BP signaling in drosophila muscles regulates organism-wide proteostasis during aging. Cell 143:813–825
Deschenes MR, Roby MA, Eason MK, Harris MB (2010) Remodeling of the neuromuscular junction precedes sarcopenia related alterations in myofibers. Exp Gerontol 45:389–393
Dirks AJ, Leeuwenburgh C (2004) Aging and lifelong calorie restriction result in adaptations of skeletal muscle apoptosis repressor, apoptosis-inducing factor, X-linked inhibitor of apoptosis, caspase-3, and caspase-12. Free Rad Biol Med 36:27–39
Dirks ML, Wall BT, Nilwik R, Weerts DH, Verdijk LB, van Loon LJ (2014) Skeletal muscle disuse atrophy is not attenuated by dietary protein supplementation in healthy older men. J Nutr 144:1196–1203
Drummond MJ, McCarthy JJ, Sinha M, Spratt HM, Volpi E, Esser KA et al (2011) Aging and microRNA expression in human skeletal muscle: a microarray and bioinformatics analysis. Physiol Genom 43:595–603
Drummond MJ, Addison O, Brunker L, Hopkins PN, McClain DA, LaStayo PC et al (2014) Downregulation of E3 ubiquitin ligases and mitophagy-related genes in skeletal muscle of physically inactive, frail older women: a cross-sectional comparison. J Gerontolol Ser A 69:1040–1048
Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A et al (2003) Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes 52:1799–1805
Evans WJ, Conley KE, Cress ME, Jubrias SA, Esselman PC, Odderson IR (1995) From muscle properties to human performance, using magnetic resonance. J Gerontol Ser A 50A:35–40
Fagiolo U, Cossarizza A, Scala E, Fanales-Belasio E, Ortolani C, Cozzi E et al (1993) Increased cytokine production in mononuclear cells of healthy elderly people European journal of Immunology 23:2375–2378
Fiatarone MA, O’Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME et al (1994) Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med 330:1769–1775
Fielding RA, LeBrasseur NK, Cuoco A, Bean J, Mizer K, Singh MAF (2002) High-velocity resistance training increases skeletal muscle peak power in older women. J Am Geriatr Soc 50:655–662
Florian MC, Nattamai KJ, Dorr K, Marka G, Uberle B, Vas V et al (2013) A canonical to non-canonical Wnt signalling switch in haematopoietic stem-cell ageing. Nature 503:392–396
Fontana L, Meyer TE, Klein S, Holloszy JO (2004) Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci USA 101:6659–6663
Francaux M, Demeulder B, Naslain D, Fortin R, Lutz O, Caty G et al (2016) Aging reduces the activation of the mTORC1 pathway after resistance exercise and protein intake in human skeletal muscle: potential role of REDD1 and impaired anabolic sensitivity. Nutrients 8:47
Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E et al (2000) Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 908:244–254
Frischer JM, Bramow S, Dal-Bianco A, Lucchinetti CF, Rauschka H, Schmidbauer M et al (2009) The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 132:1175–1189
Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL et al (2011) Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle 1:11
Fry CS, Lee JD, Mula J, Kirby TJ, Jackson JR, Liu F et al (2015) Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nature 21:76–80
Fujita S, Rasmussen B, Cadenas J, Drummond M, Glynn E, Sattler F et al (2007) Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improving endothelial function and Akt/mammalian target of rapamycin signaling. Diabetes 56:1615–1622
Fulle S, Belia S, Vecchiet J, Morabito C, Vecchiet L, Fano G (2003) Modification of the functional capacity of sarcoplasmic reticulum membranes in patients suffering from chronic fatigue syndrome. Neuromuscul Disord 13:479–484
Gaugler M, Brown A, Merrell E, DiSanto-Rose M, Rathmacher JA, Reynolds TH (2011) PKB signaling and atrogene expression in skeletal muscle of aged mice. J Appl Physiol 111:192–199
Genaro P, Pinheiro M, Szejnfeld V, Martini L (2015) Dietary protein intake in elderly women: association with muscle and bone mass. Nutr Clin Pract 30:283–289
Ghosh S, Lertwattanarak R, Lefort N, Molina-Carrion M, Joya-Galeana J, Bowen BP et al (2011) Reduction in reactive oxygen species production by mitochondria from elderly subjects with normal and impaired glucose tolerance. Diabetes 60:2051–2060
Goldspink G, Fernandes K, Williams PE, Wells DJ (1994) Age-related changes in collagen gene expression in the muscles of mdx dystrophic and normal mice. Neuromuscul Disord 4:183–191
Goljanek-Whysall K, Pais H, Rathjen T, Sweetman D, Dalmay T, Munsterberg A (2012) Regulation of multiple target genes by miR-1 and miR-206 is pivotal for C2C12 myoblast differentiation. J Cell Sci 125:3590–3600
Gouspillou G, Sgarioto N, Kapchinsky S, Purves-Smith F, Norris B, Pion CH et al (2014) Increased sensitivity to mitochondrial permeability transition and myonuclear translocation of endonuclease G in atrophied muscle of physically active older humans. FASEB J 28:1621–1633
Grassi B, Cerretelli P, Narici MV, Marconi C (1991) Peak anaerobic power in master athletes. Eur J Appl Physiol 62:394–399
Grumati P, Coletto L, Sabatelli P, Cescon M, Angelin A, Bertaggia E et al (2010) Autophagy is defective in collagen VI muscular dystrophies, and its reactivation rescues myofiber degeneration. Nat Med 16:1313–1320
Hameed M, Orrell RW, Cobbold M, Goldspink G, Harridge SD (2003) Expression of IGF-I splice variants in young and old human skeletal muscle after high resistance exercise. J Physiol 547:247–254
Hasten DL, Pak-Loduca J, Obert KA, Yarasheski KE (2000) Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78–84 and 23–32 yr olds. Am J Physiol Endocrinol Metab 278:E620–E626
Haus JM, Carrithers JA, Trappe SW, Trappe TA (2007) Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J Appl Physiol 103:2068–2076
He W, Goodkind D, Kowal P (2016) U.S. Census Bureau, international population reports, P95/16-1, an aging world: 2015. U.S. Government Publishing Office, Washington, DC
Henwood TR, Taaffe DR (2005) Improved physical performance in older adults undertaking a short-term programme of high-velocity resistance training. Gerontology 51:108–115
Hepple RT, Qin M, Nakamoto H, Goto S (2008) Caloric restriction optimizes the proteasome pathway with aging in rat plantaris muscle: implications for sarcopenia. Am J Physiol Regul Integr Comp Physiol 295:R1231–R1237
Jackson MJ, McArdle A (2016) Role of reactive oxygen species in age-related neuromuscular deficits. J Physiol 594:1979–1988
Jackson J, Mula J, Kirby TJ, Fry CS, Lee JD, Ubele MF et al (2012) Satellite cell depletion does not inhibit adult skeletal muscle regrowth following unloading-induced atrophy. Am J Physiol Cell Physiol 303:C854–C861
Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R (2004) The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc 52:80–85
Jiang JC, Jaruga E, Repnevskaya MV, Jazwinski SM (2000) An intervention resembling caloric restriction prolongs life span and retards aging in yeast. FASEB J 14:2135–2137
Joseph A-M, Malamo AG, Silvestre J, Wawrzyniak N, Carey-Love S, Nguyen LMD et al (2013a) Short-term caloric restriction, resveratrol, or combined treatment regimens initiated in late-life alter mitochondrial protein expression profiles in a fiber-type specific manner in aged animals. Exp Gerontol 48:858–868
Joseph AM, Adhihetty PJ, Wawrzyniak NR, Wohlgemuth SE, Picca A, Kujoth GC et al (2013b) Dysregulation of mitochondrial quality control processes contribute to sarcopenia in a mouse model of premature aging. PLoS ONE 8:e69327
Kadhiresan VA, Hassett CA, Faulkner JA (1996) Properties of single motor units in medial gastrocnemius muscles of adult and old rats. J Physiol 493:543–552
Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR (2006) A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Endocrinol Metab 291:E381–E387
Kayani AC, Close GL, Jackson MJ, McArdle A (2008) Prolonged treadmill training increases HSP70 in skeletal muscle but does not affect age-related functional deficits. Am J Physiol Regul Integr Comp Physiol 294:R568–R576
Kemmler W, Schliffka R, Mayhew JL, von Stengel S (2010) Effects of whole-body electromyostimulation on resting metabolic rate, body composition, and maximum strength in postmenopausal women: the training and electrostimulation trial. J Strength Cond Res 24:1880–1887
Kemmler W, Bebenek M, Engelke K, von Stengel S (2014) Impact of whole-body electromyostimulation on body composition in elderly women at risk for sarcopenia: the training and electrostimulation trial (TEST-III). Age 36:395–406
Kiffin R, Kaushik S, Zeng M, Bandyopadhyay U, Zhang C, Massey AC et al (2007) Altered dynamics of the lysosomal receptor for chaperone-mediated autophagy with age. J Cell Sci 120:782–791
Kohut ML, McCann DA, Russell DW, Konopka DN, Cunnick JE, Franke WD et al (2006) Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of β-blockers, BMI, and psychosocial factors in older adults. Brain Behav Immun 20:201–209
Konopka A, Suer M, Wolff C, Harber MP (2013) Markers of human skeletal muscle mitochondrial biogenesis and quality control: effects of age and aerobic exercise training. J Biogerontol 69:371–378
Koopman R, Walrand S, Beelen M, Gijsen AP, Kies AK, Boirie Y et al (2009) Dietary protein digestion and absorption rates and the subsequent postprandial muscle protein synthetic response do not differ between young and elderly men. J Nutr 139:1707–1713
Korovila I, Hugo M, Castro JP, Weber D, Hohn A, Grune T et al (2017) Proteostasis, oxidative stress and aging. Redox Biol 13:550–567
Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE et al (2005) Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309:481–484
Kurosaka M, Machida S (2013) Interleukin-6-induced satellite cell proliferation is regulated by induction of the JAK2/STAT3 signalling pathway through cyclin D1 targeting. Cell Prolif 46:365–373
Lagrand WK, Visser CA, Hermens WT, Niessen HWM, Verheugt FWA, Wolbink G-J et al (1999) C-reactive protein as a cardiovascular risk factor: more than an epiphenomenon? Circulation 100:96–102
Lakowski B, Hekimi S (1989) The genetics of caloric restriction in Caenorhabditis elegans. PNAS 95:13091–13096
Larkin LM, Davis CS, Sims-Robinson C, Kostrominova TY, Remmen HV, Richardson A et al (2011) Skeletal muscle weakness due to deficiency of CuZn-superoxide dismutase is associated with loss of functional innervation. Am J Physiol Regul Integr Comp Physiol 301:R1400–R1407
Larsson L (1995) Motor units: remodeling in aged animals. J Gerontol Ser A 50A:91–95
Larsson L, Sjödin B, Karlsson J (1978) Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22–65 years. Acta Physiol Scand 103:31–39
Leduc-Gaudet JP, Picard M, St-Jean Pelletier F, Sgarioto N, Auger MJ, Vallee J et al (2015) Mitochondrial morphology is altered in atrophied skeletal muscle of aged mice. Oncotarget 6:17923–17937
Lee S-J (2007) Quadrupling muscle mass in mice by targeting TGF-ß signaling pathways. PLoS ONE 2:e789
Lee CM, Lopez ME, Weindruch R, Aiken JM (1998a) Association of age-related mitochondrial abnormalities with skeletal muscle fiber atrophy. Free Rad Biol Med 25:964–972
Lee CM, Aspnes LE, Chung SS, Weindruch R, Aiken JM (1998b) Influences of caloric restriction on age-associated skeletal muscle fiber characteristics and mitochondrial changes in rats and Micea. Ann N Y Acad Sci 854:182–191
Lee WS, Cheung WH, Qin L, Tang N, Leung KS (2006) Age-associated decrease of type IIA/B human skeletal muscle fibers. Clin Orthop Relat Res 450:231–237
Lee JS, Auyeung TW, Kwok T, Lau EM, Leung PC, Woo J (2007) Associated factors and health impact of sarcopenia in older chinese men and women: a cross-sectional study. Gerontology 53:404–410
Lee HY, Choi CS, Birkenfeld AL, Alves TC, Jornayvaz FR, Jurczak MJ et al (2010) Targeted expression of catalase to mitochondria prevents age-associated reductions in mitochondrial function and insulin resistance. Cell Metab 12:668–674
Lee ASJ, Anderson JE, Joya JE, Head SI, Pather N, Kee AJ et al (2013) Aged skeletal muscle retains the ability to fully regenerate functional architecture. BioArchitecture 3:25–37
Leenders M, Verdijk LB, van der Hoeven L, van Kranenburg J, Nilwik R, van Loon LJ (2013) Elderly men and women benefit equally from prolonged resistance-type exercise training. J Gerontol 68:769–779
Levinger I, Howlett KF, Peake J, Garnham A, Hare DL, Jerums G et al (2010) Akt, AS160, metabolic risk factors and aerobic fitness in middle-aged women. Exerc Immunol Rev 16:98–104
Lexell J, Taylor CC, Sjöström M (1988) What is the cause of the ageing atrophy?: total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 84:275–294
Li YP (2003) TNF-alpha is a mitogen in skeletal muscle. Am J Cell Physiol 285:C370–C376
Li YP, Schwartz RJ, Waddell ID, Holloway BR, Reid MB (1998) Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NF-kappaB activation in response to tumor necrosis factor alpha. FASEB J 12:871–880
Lightfoot AP, Sakellariou GK, Nye GA, McArdle F, Jackson MJ, Griffiths RD et al (2015) SS-31 attenuates TNF-alpha induced cytokine release from C2C12 myotubes. Redox Biol 6:253–259
Ling SM, Conwit RA, Ferrucci L, Metter EJ (2009) Age-associated changes in motor unit physiology: observations from the Baltimore longitudinal study of aging. Arch Phys Med Rehabil 90:1237–1240
Liu CJ, Latham NK (2009) Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev 3:CD002759
Liu W, Klose A, Forman S, Paris ND, Wei-LaPierre L, Cortes-Lopez M et al (2017) Loss of adult skeletal muscle stem cells drives age-related neuromuscular junction degeneration. Elife 6:e26464
Logan A, Shabalina IG, Prime TA, Rogatti S, Kalinovich AV, Hartley RC et al (2014) In vivo levels of mitochondrial hydrogen peroxide increase with age in mtDNA mutator mice. Aging Cell 13:765–768
Maltais ML, Perreault-Ladouceur J, Dionne IJ (2015) The effect of resistance training and different sources of post-exercise protein supplementation on muscle mass and physical capacity in sarcopenic elderly men. J Strength Cond Res 30:1680–1687
Marzetti E, Wohlgemuth SE, Lees HA, Chung HY, Giovannini S, Leeuwenburgh C (2008) Age-related activation of mitochondrial caspase-independent apoptotic signaling in rat gastrocnemius muscle. Mech Ageing Dev 129:542–549
McMahon CD, Chai R, Radley-Crabb HG, Watson T, Matthews KG, Sheard PW et al (2014) Lifelong exercise and locally produced insulin-like growth factor-1 (IGF-1) have a modest influence on reducing age-related muscle wasting in mice. Scand J Med Sci Sports 24:e423–e435
Mecocci P, Fano G, Fulle S, MacGarvey U, Shinobu L, Polidori MC et al (1999) Age-dependent increases in oxidative damage to DNA, lipids, and proteins in human skeletal muscle. Free Rad Biol Med 26:303–308
Mercken EM, Crosby SD, Lamming DW, JeBailey L, Krzysik-Walker S, Villareal DT et al (2013) Calorie restriction in humans inhibits the PI3 K/AKT pathway and induces a younger transcription profile. Aging Cell 12:645–651
Minet AD, Gaster M (2012) Cultured senescent myoblasts derived from human vastus lateralis exhibit normal mitochondrial ATP synthesis capacities with correlating concomitant ROS production while whole cell ATP production is decreased. Biogerontology 13:277–285
Miquel J, Economos AC, Fleming J, Johnson JE Jr (1980) Mitochondrial role in cell aging. Exp Gerontol 15:575–591
Mitchell D, Haan MN, Steinberg FM, Visser M (2003) Body composition in the elderly: the influence of nutritional factors and physical activity. J Nutr Health Aging 7:130–139
Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD et al (2015) Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol Ser A 70:57–62
Mosoni L, Gatineau E, Gatellier P, Migne C, Savary-Auzeloux I, Remond D et al (2014) High whey protein intake delayed the loss of lean body mass in healthy old rats, whereas protein type and polyphenol/antioxidant supplementation had no effects. PLoS ONE 9:e109098
Muller FL, Song W, Liu Y, Chaudhuri A, Pieke-Dahl S, Strong R et al (2006) Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Rad Biol Med 40:1993–2004
Murphy Malea M, Keefe Alexandra C, Lawson Jennifer A, Flygare Steven D, Yandell M, Kardon G (2014) Transiently active Wnt/β-catenin signaling is not required but must be silenced for stem cell function during muscle regeneration. Stem Cell Rep 3:475–488
Nilwik R, Snijders T, Leenders M, Groen BB, van Kranenburg J, Verdijk LB et al (2013) The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp Gerontol 48:492–498
Norman K, Stobaus N, Kulka K, Schulzke J (2014) Effect of inflammation on handgrip strength in the non-critically ill is independent from age, gender and body composition. Eur J Clin Nutr 68:155–158
Norton C, Toomey C, McCormack WG, Francis P, Saunders J, Kerin E et al (2015) Protein supplementation at breakfast and lunch for 24 weeks beyond habitual intakes increases whole-body lean tissue mass in healthy older adults. J Nutr 146:65–69
Oumi M, Miyoshi M, Yamamoto T (2000) The ultrastructure of skeletal and smooth muscle in experimental protein malnutrition in rats fed a low protein diet. Arch Histol Cytol 63:451–457
Palomero J, Vasilaki A, Pye D, McArdle A, Jackson MJ (2013) Aging increases the oxidation of dichlorohydrofluorescein in single isolated skeletal muscle fibers at rest, but not during contractions. Am J Physiol 305:R351–R358
Palop M, Parraga J, Lozana AE, Arteaga C (2015) Sarcopenia intervention with progressive resistance training and protein nutritional supplements. Nutr Hosp 31:1481–1490
Pedersen M, Bruunsgaard H, Weis N, Hendel H, Andreassen B, Eldrup E et al (2003) Circulating levels of TNF-alpha and IL-6-relation to truncal fat mass and muscle mass in healthy elderly individuals and in patients with type-2 diabetes. Mech Ageing Dev 124:495–502
Pereira MG, Silva MT, da Cunha FM, Moriscot AS, Aoki MS, Miyabara EH (2015) Leucine supplementation improves regeneration of skeletal muscles from old rats. Exp Gerontol 72:269–277
Pfeifer U, Warmuth-Metz M (1983) Inhibition by insulin of cellular autophagy in proximal tubular cells of rat kidney. Am J Physiol 244:E109–E114
Phillips T, Leeuwenburgh C (2005) Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB J 19:668–670
Piasecki M, Ireland A, Stashuk D, Hamilton-Wright A, Jones DA, McPhee JS (2015) Age-related neuromuscular changes affecting human vastus lateralis. J Physiol 594:4525–4536
Pierik VD, Meskers CGM, Van Ancum JM, Numans ST, Verlaan S, Scheerman K et al (2017) High risk of malnutrition is associated with low muscle mass in older hospitalized patients - a prospective cohort study. BMC Geriatr 17:118
Pinti M, Cevenini E, Nasi M, De Biasi S, Salvioli S, Monti D et al (2014) Circulating mitochondrial DNA increases with age and is a familiar trait: implications for “inflamm-aging”. Eur J Immunol 44:1552–1562
Pisani DF, Clement N, Loubat A, Plaisant M, Sacconi S, Kurzenne JY et al (2010) Hierarchization of myogenic and adipogenic progenitors within human skeletal muscle. Stem Cells 28:2182–2194
Raguso CA, Kyle U, Kossovsky MP, Roynette C, Paoloni-Giacobino A, Hans D et al (2006) A 3-year longitudinal study on body composition changes in the elderly: role of physical exercise. Clin Nutr 25:573–580
Ramaswamy KS, Palmer ML, van der Meulen JH, Renoux A, Kostrominova TY, Michele DE et al (2011) Lateral transmission of force is impaired in skeletal muscles of dystrophic mice and very old rats. J Physiol 589:1195–1208
Rando TA, Crowley RS, Carlson EJ, Epstein CJ, Mohapatra PK (1998) Overexpression of copper/zinc superoxide dismutase: a novel cause of murine muscular dystrophy. Ann Neurol 44:381–386
Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S (2006) Myogenic gene expression at rest and after a bout of resistance exercise in young (18-30 yr) and old (80-89 yr) women. J Appl Physiol 1985(101):53–59
Reid KF, Callahan DM, Carabello RJ, Phillips EM, Frontera WR, Fielding RA (2008) Lower extremity power training in elderly subjects with mobility limitations: a randomized controlled trial. Aging Clin Exp Res 20:337–343
Ribeiro MBT, Guzzoni V, Hord JM, Lopes GN, Marqueti RC, de Andrade RV et al (2017) Resistance training regulates gene expression of molecules associated with intramyocellular lipids, glucose signaling and fiber size in old rats. Sci Rep 7:8593
Rosenberg I (1989) Summary comments: epidemiological and methodological problems in determining nutritional status of older persons. Am J Clin Nutr 50:1231–1233
Russ DW, Krause J, Wills A, Arreguin R (2012) “SR stress” in mixed hindlimb muscles of aging male rats. Biogerontology 13:547–555
Russ DW, Boyd IM, McCoy KM, McCorkle KW (2015a) Muscle-specificity of age-related changes in markers of autophagy and sphingolipid metabolism. Biogerontology 16:747–759
Russ DW, Acksel C, Boyd IM, Maynard J, McCorkle KW, Edens NK et al (2015b) Dietary HMB and beta-alanine co-supplementation does not improve in situ muscle function in sedentary, aged male rats. Appl Physiol Nutr Metab 40:1294–1301
Russell AP, Lamon S, Boon H, Wada S, Güller I, Brown EL et al (2013) Regulation of miRNAs in human skeletal muscle following acute endurance exercise and short-term endurance training. J Physiol 591:4637–4653
Ryan MJ, Dudash HJ, Docherty M, Geronilla KB, Baker BA, Haff GG et al (2008) Aging-dependent regulation of antioxidant enzymes and redox status in chronically loaded rat dorsiflexor muscles. J Gerontol Ser A 63:1015–1026
Sacheck J, Philippe J, Hyatt K, Raffaello A, Thomas Jagoe R, Roy R et al (2007) Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J 21:140–155
Sakellariou GK, Vasilaki A, Palomero J, Kayani A, Zibrik L, McArdle A et al (2013) Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxid Redox Signal 18:603–621
Sakellariou GK, Jackson MJ, Vasilaki A (2014a) Redefining the major contributors to superoxide production in contracting skeletal muscle. The role of NAD(P)H oxidases. Free Rad Res 48:12–29
Sakellariou GK, Davis CS, Shi Y, Ivannikov MV, Zhang Y, Vasilaki A et al (2014b) Neuron-specific expression of CuZnSOD prevents the loss of muscle mass and function that occurs in homozygous CuZnSOD-knockout mice. FASEB J 28:1666–1681
Sakellariou GK, Pearson T, Lightfoot AP, Nye GA, Wells N, Giakoumaki II et al (2016) Mitochondrial ROS regulate oxidative damage and mitophagy but not age-related muscle fiber atrophy. Sci Rep 6:33944
Sandiford SD, Kennedy KA, Xie X, Pickering JG, Li SS (2014) Dual oxidase maturation factor 1 (DUOXA1) overexpression increases reactive oxygen species production and inhibits murine muscle satellite cell differentiation. Cell Commun Signal 12:5
Sato T, Ito Y, Nagasawa T (2013) Regulation of skeletal muscle protein degradation and synthesis by oral administration of lysine in rats. J Nutr Sci Vitaminol 59:412–419
Sato T, Ito Y, Nedachi T, Nagasawa T (2014) Lysine suppresses protein degradation through autophagic-lysosomal system in C2C12 myotubes. Mol Cell Biochem 391:37–46
Sato T, Ito Y, Nagasawa T (2015) Dietary l-lysine suppresses autophagic proteolysis and stimulates Akt/mTOR signaling in the skeletal muscle of rats fed a low-protein diet. J Agric Food Chem 63:8192–8198
Schaap LA, Pluijm SM, Deeg DJ, Visser M (2006) Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med 119:526 e529-517
Schaap LA, Pluijm SM, Deeg DJ, Harris TB, Kritchevsky SB, Newman AB et al (2009) Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol Ser A 64:1183–1189
Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z (2007) Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J 26:1749–1760
Schulte JN, Yarasheski KE (2001) Effects of resistance training on the rate of muscle protein synthesis in frail elderly people. Int J Sport Nutr Exerc Metab 11(Suppl):S111–S118
Schwartz RS, Shuman WP, Larson V, Cain KC, Fellingham GW, Beard JC et al (1991) The effect of intensive endurance exercise training on body fat distribution in young and older men. Metabolism 40:545–551
Serrano AL, Munoz-Canoves P (2010) Regulation and dysregulation of fibrosis in skeletal muscle. Exp Cell Res 316:3050–3058
Shafiq SA, Gorycki MA (1965) Regeneration in skeletal muscle of mouse: some electron-microscope observations. J Pathol Bacteriol 90:123–127
Shahar S, Kamaruddin NS, Badrasawi M, Sakian NIM, Manaf ZA, Yassin Z et al (2013) Effectiveness of exercise and protein supplementation intervention on body composition, functional fitness, and oxidative stress among elderly Malays with sarcopenia. Clin Interv Aging 8:1365–1375
Shefer G, Van de Mark D, Richardson J, Yablonka-Reuveni Z (2006) Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Ageing Cell 294:50–66
Sheth KA, Iyer CC, Wier CG, Crum AE, Bratasz A, Kolb SJ et al (2018) Muscle strength and size are associated with motor unit connectivity in aged mice. Neurobiol Aging 67:128–136
Short K, Vittone J, Bigelow M, Proctor D, Rizza R, Coenen-Shimke J et al (2003) Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes 52:1888–1896
Short K, Bigelow M, Kahl J, Singh R, Coenen-Schimke J (2005) Decline in skeletal muscle mitochondrial function with aging in humans. PNAS 102:5618–5623
Smeuninx B, McKendry J, Wilson D, Martin U, Breen L (2017) Age-related anabolic resistance of myofibrillar protein synthesis is exacerbated in obese inactive individuals. J Clin Endocrinol and Metab 102:3535–3545
Song MY, Ruts E, Kim J, Janumala I, Heymsfield S, Gallagher D (2004) Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am J Clin Nutr 79:874–880
Soriano-Arroquia A, McCormick R, Molloy AP, McArdle A, Goljanek-Whysall K (2016a) Age-related changes in miR-143-3p:Igfbp5 interactions affect muscle regeneration. Aging Cell 15:361–369
Soriano-Arroquia A, House L, Tregilgas L, Canty-Laird E, Goljanek-Whysall K (2016b) The functional consequences of age-related changes in microRNA expression in skeletal muscle. Biogerontology 17:641–654
Sousa-Victor P, Gutarra S, Garcia-Prat L, Rodriguez-Ubreva J, Ortet L, Ruiz-Bonilla V et al (2014) Geriatric muscle stem cells switch reversible quiescence into senescence. Nature 506:316–321
Stárka L (2006) Testosteron treatment in sarcopenia. Vnitr Lekarstvi 52:909–911
Stengel S, Bebenek M, Engelke K, Kemmler W (2015) Whole-body electromyostimulation to fight osteopenia in elderly females: the randomized controlled training and electrostimulation trial (TEST-III). J Oesteoporosis. https://doi.org/10.1155/2015/643520
Sullivan-Gunn M, Lewandowski P (2013) Elevated hydrogen peroxide and decreased catalase and glutathione peroxidase protection are associated with aging sarcopenia. BMC Geriatr 13:104
Symons TB, Schutzler SE, Cocke TL, Chinkes DL, Wolfe RR, Paddon-Jones D (2007) Aging does not impair the anabolic response to a protein-rich meal. Am J Clin Nutr 86:451–456
Taaffe DR, Duret C, Wheeler S, Marcus R (1999) Once-weekly resistance exercise improves muscle strength and neuromuscular performance in older adults. J Am Geriatr Soc 47:1208–1214
Tarry-Adkins JL, Fernandez-Twinn DS, Chen JH, Hargreaves IP, Neergheen V, Aiken CE et al (2016) Poor maternal nutrition and accelerated postnatal growth induces an accelerated aging phenotype and oxidative stress in skeletal muscle of male rats. Dis Model Mech 9:1221–1229
Thalacker-Mercer AE, Dell’Italia LJ, Cui X, Cross JM, Bamman MM (2010) Differential genomic responses in old vs. young humans despite similar levels of modest muscle damage after resistance loading. Physiol Genom 40:141–149
Thannickal VJ, Fanburg BL (2000) Reactive oxygen species in cell signaling. Lung Cell Mol Physiol 279:L1005–L1028
Tiainen K, Hurme M, Hervonen A, Luukkaala T, Jylha M (2010) Inflammatory markers and physical performance among nonagenarians. J Gerontol Ser A 65:658–663
Tieland M, Dirks ML, van der Zwaluw N, Verdijk LB, van de Rest O, de Groot LC et al (2012) Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 13:713–719
Tomlinson BE, Irving D (1977) The numbers of limb motor neurons in the human lumbosacral cord throughout life. J Neurol Sci 34:213–219
Tsuzuku S, Kajioka T, Sakakibara H, Shimaoka K (2018) Slow movement resistance training using body weight improves muscle mass in the elderly: a randomized controlled trial. Scand J Med Sci Sports 28:1339–1344
Valenzuela PL, Morales JS, Pareja-Galeano H, Izquierdo M, Emanuele E, de la Villa P et al (2018) Physical strategies to prevent disuse-induced functional decline in the elderly. Ageing Res Rev 47:80–88
Vasilaki A, Mansouri A, Van Remmen H, van der Meulen JH, Larkin L, Richardson AG et al (2006) Free radical generation by skeletal muscle of adult and old mice: effect of contractile activity. Aging Cell 5:109–117
Vasilaki A, van der Meulen JH, Larkin L, Harrison DC, Pearson T, Van Remmen H et al (2010) The age-related failure of adaptive responses to contractile activity in skeletal muscle is mimicked in young mice by deletion of Cu, Zn superoxide dismutase. Aging Cell 9:979–990
Vasilaki A, Pollock N, Giakoumaki I, Goljanek-Whysall K, Sakellariou GK, Pearson T et al (2016) The effect of lengthening contractions on neuromuscular junction structure in adult and old mice. Age 38:259–272
Verdijk LB, Gleeson B, Jonkers R, Meijer K, Savelberg H, Dendale P et al (2009a) Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J Biogerontol 64A:332–339
Verdijk LB, Jonkers RA, Gleeson BG, Beelen M, Meijer K, Savelberg HH et al (2009b) Protein supplementation before and after exercise does not further augment skeletal muscle hypertrophy after resistance training in elderly men. Am J Clin Nutr 89:608–616
Verdijk LB, Snijders T, Drost M, Delhaas T, Kadi F, van Loon LJC (2014) Satellite cells in human skeletal muscle; from birth to old age. Age 36:545–557
Verreijen AM, Verlaan S, Engberink MF, Swinkels S, de Vogel-van den Bosch J, Weijs PJ (2015) A high whey protein-, leucine-, and vitamin D-enriched supplement preserves muscle mass during intentional weight loss in obese older adults: a double-blind randomized controlled trial. Am J Clin Nutr 101:279–286
Vertino AM, Taylor-Jones JM, Longo KA, Bearden ED, Lane TF, McGehee RE et al (2005) Wnt10b deficiency promotes coexpression of myogenic and adipogenic programs in myoblasts. Mol Biol Cell 16:2039–2048
Vettor R, Milan G, Franzin C, Sanna M, De Coppi P, Rizzuto R et al (2009) The origin of intermuscular adipose tissue and its pathophysiological implications. Am J Physiol Endocrinol Metab 297:E987–E998
Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR (2001) Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA 286:1206–1212
Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR (2003) Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr 78:250–258
Wall BT, Gorissen SH, Pennings B, Koopman R, Groen BB, Verdijk LB et al (2015) Aging is accompanied by a blunted muscle protein synthetic response to protein ingestion. PLoS ONE 10:e0140903
Walrand S, Short KR, Bigelow ML, Sweatt AJ, Hutson SM, Nair KS (2008) Functional impact of high protein intake on healthy elderly people. Am J Physiol Endocrinol Metab 295:E921–E928
Wanagat J, Cao Z, Pathare P, Aiken JM (2001) Mitochondrial DNA deletion mutations colocalize with segmental electron transport system abnormalities, muscle fiber atrophy, fiber splitting, and oxidative damage in sarcopenia. FASEB J 15:322–332
Wang Y, Wehling-Henricks M, Samengo G, Tidball JG (2015) Increases of M2a macrophages and fibrosis in aging muscle are influenced by bone marrow aging and negatively regulated by muscle-derived nitric oxide. Aging Cell 14:678–688
Weindruch R, Walford R, Fligiel S, Guthrie A (1986) The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr 116:614–654
Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB et al (2006) Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr 84:1033–1042
Welle S, Thornton C, Jozefowicz R, Statt M (1993) Myofibrillar protein synthesis in young and old men. Am J Physiol 264:693–698
Wroblewski AP, Amati F, Smiley MA, Goodpaster B, Wright V (2011) Chronic exercise preserves lean muscle mass in masters athletes. Phys Sportsmed 39:172–178
Xu Z, Tan Z, Zhang Q, Gui Q, Yang T (2014) Clinical effectiveness of protein and amino acid supplementation on building muscle mass in elderly people: a meta-analysis. PLoS ONE 9:e109141
Yoon YS, Yoon DS, Lim IK, Yoon SH, Chung HY, Rojo M et al (2006) Formation of elongated giant mitochondria in DFO-induced cellular senescence: involvement of enhanced fusion process through modulation of Fis1. J Cell Physiol 209:468–480
Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA, Beauchamp JR (2004) Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J Cell Biol 166:347–357
Zhu K, Kerr DA, Meng X, Devine A, Solah V, Binns CW et al (2015) Two-year whey protein supplementation did not enhance muscle mass and physical function in well-nourished healthy older postmenopausal women. J Nutr 145:2520–2526
Acknowledgements
The authors would like to thanks Professor Anne McArdle for her input in this review. The authors’ work is supported by the Biotechnology and Biological Sciences Research Council (BBSRC). Due to space constraints, the authors would like to apologise to fellow colleagues whose work was not cited in this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
McCormick, R., Vasilaki, A. Age-related changes in skeletal muscle: changes to life-style as a therapy. Biogerontology 19, 519–536 (2018). https://doi.org/10.1007/s10522-018-9775-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-018-9775-3