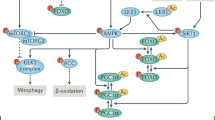
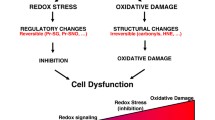
Abstract
“Organ reserve” refers to the ability of an organ to successfully return to its original physiological state following repeated episodes of stress. Clinical evidence shows that organ reserve correlates with the ability of older adults to cope with an added workload or stress, suggesting a role in the process of aging. Although organ reserve is well documented clinically, it is not clearly defined at the molecular level. Interestingly, several metabolic pathways exhibit excess metabolic capacities (e.g., bioenergetics pathway, antioxidants system, plasticity). These pathways comprise molecular components that have an excess of quantity and/or activity than that required for basic physiological demand in vivo (e.g., mitochondrial complex IV or glycolytic enzymes). We propose that the excess in mtDNA copy number and tandem DNA repeats of telomeres are additional examples of intrinsically embedded structural components that could comprise excess capacity. These excess capacities may grant intermediary metabolism the ability to instantly cope with, or manage, added workload or stress. Therefore, excess metabolic capacities could be viewed as an innate mechanism of adaptability that substantiates organ reserve and contributes to the cellular defense systems. If metabolic excess capacities or organ reserves are impaired or exhausted, the ability of the cell to cope with stress is reduced. Under these circumstances cell senescence, transformation, or death occurs. In this review, we discuss excess metabolic and structural capacities as integrated metabolic pathways in relation to organ reserve and cellular aging.



Similar content being viewed by others
Abbreviations
- mtDNA:
-
Mitochondrial DNA
- Complex IV:
-
Cytochrome c oxidase
- HMS:
-
Hexose monophosphate shunt
- TCA:
-
Tricarboxylic acids cycle
- ETC`:
-
Electron transport chain
References
Ahles TA, Root JC, Ryan EL (2012) Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J Clin Oncol 30:3675–3686
Aiken CE, Tarry-Adkins JL, Ozanne SE (2015) Transgenerational developmental programming of ovarian reserve. Sci Rep 5:16175
Atamna H, Atamna W, Al-Eyd G et al (2015) Combined activation of the energy and cellular-defense pathways may explain the potent anti-senescence activity of methylene blue. Redox Biol 6:426–435
Bernadotte A, Mikhelson VM, Spivak IM (2016) Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging (Albany NY) 8:3–11
Bolanos JP, Almeida A, Moncada S (2010) Glycolysis: a bioenergetic or a survival pathway? Trends Biochem Sci 35:145–149
Bortz WMT, Bortz WM 2nd (1996) How fast do we age? Exercise performance over time as a biomarker. J Gerontol A Biol Sci Med Sci 51:M223–M225
Brand MD (2016) Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic Biol Med 100:14–31
Calado RT, Dumitriu B (2013) Telomere dynamics in mice and humans. Semin Hematol 50:165–174
Callen E, Surralles J (2004) Telomere dysfunction in genome instability syndromes. Mutat Res 567:85–104
Carnes BA, Witten TM (2014) How long must humans live? J Gerontol A Biol Sci Med Sci 69:965–970
Choi SW, Gerencser AA, Nicholls DG (2009) Bioenergetic analysis of isolated cerebrocortical nerve terminals on a microgram scale: spare respiratory capacity and stochastic mitochondrial failure. J Neurochem 109:1179–1191
D’adda Di Fagagna F, Reaper PM, Clay-Farrace L, et al (2003) A DNA damage checkpoint response in telomere-initiated senescence. Nature 426:194–198
Davey GP, Clark JB (1996) Threshold effects and control of oxidative phosphorylation in nonsynaptic rat brain mitochondria. J Neurochem 66:1617–1624
De Lores Arnaiz GR, Ordieres MG (2014) Brain na(+), k(+)-atpase activity in aging and disease. Int J Biomed Sci 10:85–102
Demetrius L, Legendre S, Harremoes P (2009) Evolutionary entropy: a predictor of body size, metabolic rate and maximal life span. Bull Math Biol 71:800–818
Desler C, Hansen TL, Frederiksen JB et al (2012) Is there a link between mitochondrial reserve respiratory capacity and aging? J Aging Res 2012:192503
Diamond J, Hammond K (1992) The matches, achieved by natural selection, between biological capacities and their natural loads. Experientia 48:551–557
Dranka BP, Hill BG, Darley-Usmar VM (2010) Mitochondrial reserve capacity in endothelial cells: the impact of nitric oxide and reactive oxygen species. Free Radic Biol Med 48:905–914
Eanes WF, Merritt TJ, Flowers JM et al (2006) Flux control and excess capacity in the enzymes of glycolysis and their relationship to flight metabolism in drosophila melanogaster. Proc Natl Acad Sci USA 103:19413–19418
Ferguson-Miller S, Hiser C, Liu J (2012) Gating and regulation of the cytochrome c oxidase proton pump. Biochim Biophys Acta 1817:489–494
Fumagalli M, Rossiello F, Clerici M et al (2012) Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat Cell Biol 14:355–365
Fumagalli M, Rossiello F, Mondello C, D’adda Di Fagagna F (2014) Stable cellular senescence is associated with persistent ddr activation. PLoS ONE 9:e110969
Gardner M, Bann D, Wiley L et al (2014) Gender and telomere length: systematic review and meta-analysis. Exp Gerontol 51:15–27
Gladyshev VN (2016) Aging: progressive decline in fitness due to the rising deleteriome adjusted by genetic, environmental, and stochastic processes. Aging Cell 15:594–602
Gnaiger E, Lassnig B, Kuznetsov A et al (1998) Mitochondrial oxygen affinity, respiratory flux control and excess capacity of cytochrome c oxidase. J Exp Biol 201:1129–1139
Goldspink DF (2005) Ageing and activity: their effects on the functional reserve capacities of the heart and vascular smooth and skeletal muscles. Ergonomics 48:1334–1351
Gong G, Liu J, Liang P et al (2003) Oxidative capacity in failing hearts. Am J Physiol Heart Circ Physiol 285:H541–H548
Guidot DM, Mccord JM, Wright RM, Repine JE (1993) Absence of electron transport (rho 0 state) restores growth of a manganese-superoxide dismutase-deficient saccharomyces cerevisiae in hyperoxia. Evidence for electron transport as a major source of superoxide generation in vivo. J Biol Chem 268:26699–26703
Harrison DE, Strong R, Allison DB et al (2014) Acarbose, 17-alpha-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell 13:273–282
Hayflick L (2007) Entropy explains aging, genetic determinism explains longevity, and undefined terminology explains misunderstanding both. PLoS Genet 3:e220
Henle ES, Han Z, Tang N et al (1999) Sequence-specific DNA cleavage by fe2+-mediated fenton reactions has possible biological implications. J Biol Chem 274:962–971
Herbst A, Wanagat J, Cheema N et al (2016) Latent mitochondrial DNA deletion mutations drive muscle fiber loss at old age. Aging Cell 15(6):1132–1139
Herrera A, Garcia I, Gaytan N et al (2015) Endangered species: mitochondrial DNA loss as a mechanism of human disease. Front Biosci (Schol Ed) 7:109–124
Hill BG, Dranka BP, Zou L et al (2009) Importance of the bioenergetic reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. Biochem J 424:99–107
Houtkooper RH, Argmann C, Houten SM et al (2011) The metabolic footprint of aging in mice. Sci Rep 1:134
Howarth C, Gleeson P, Attwell D (2012) Updated energy budgets for neural computation in the neocortex and cerebellum. J Cereb Blood Flow Metab 32:1222–1232
Iliodromiti S, Iglesias C, Messow CM et al (2016) Excessive age-related decline in functional ovarian reserve in infertile women: prospective cohort of 15,500 women. J Clin Endocrinol Metab 101(9):3548–3554
Jenkins NL, Mccoll G, Lithgow GJ (2004) Fitness cost of extended lifespan in caenorhabditis elegans. Proc Biol Sci 271:2523–2526
Jeyapalan JC, Ferreira M, Sedivy JM, Herbig U (2007) Accumulation of senescent cells in mitotic tissue of aging primates. Mech Ageing Dev 128:36–44
Jones TT, Brewer GJ (2010) Age-related deficiencies in complex i endogenous substrate availability and reserve capacity of complex iv in cortical neuron electron transport. Biochim Biophys Acta 1797:167–176
Kamath RS, Fraser AG, Dong Y et al (2003) Systematic functional analysis of the caenorhabditis elegans genome using rnai. Nature 421:231–237
Kingsley-Hickman PB, Sako EY, Ugurbil K et al (1990) 31p nmr measurement of mitochondrial uncoupling in isolated rat hearts. J Biol Chem 265:1545–1550
Klichko V, Sohal BH, Radyuk SN et al (2014) Decrease in cytochrome c oxidase reserve capacity diminishes robustness of drosophila melanogaster and shortens lifespan. Biochem J 459:127–135
Knudsen T, Johansen T (1990) Regulation of the na(+)-k+ pump activity and estimation of the reserve capacity in intact rat peritoneal mast cells. FEBS Lett 269:7–10
Kogelnik AM, Lott MT, Brown MD et al (1998) Mitomap: a human mitochondrial genome database–1998 update. Nucleic Acids Res 26:112–115
Kong CM, Lee XW, Wang X (2013) Telomere shortening in human diseases. FEBS J 280:3180–3193
Kramer PA, Ravi S, Chacko B et al (2014) A review of the mitochondrial and glycolytic metabolism in human platelets and leukocytes: implications for their use as bioenergetic biomarkers. Redox Biol 2:206–210
Lanza IR, Zabielski P, Klaus KA et al (2012) Chronic caloric restriction preserves mitochondrial function in senescence without increasing mitochondrial biogenesis. Cell Metab 16:777–788
Lapointe J, Hekimi S (2008) Early mitochondrial dysfunction in long-lived mclk1 ± mice. J Biol Chem 283:26217–26227
Lee HC, Wei YH (2005) Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. Int J Biochem Cell Biol 37:822–834
Liu L, Trimarchi JR, Smith PJ, Keefe DL (2002) Mitochondrial dysfunction leads to telomere attrition and genomic instability. Aging Cell 1:40–46
Madonna R, De Caterina R, Willerson JT, Geng YJ (2011) Biologic function and clinical potential of telomerase and associated proteins in cardiovascular tissue repair and regeneration. Eur Heart J 32:1190–1196
Manke T, Demetrius L, Vingron M (2006) An entropic characterization of protein interaction networks and cellular robustness. J R Soc Interface 3:843–850
Mao P, Reddy PH (2011) Aging and amyloid beta-induced oxidative DNA damage and mitochondrial dysfunction in Alzheimer’s disease: implications for early intervention and therapeutics. Biochim Biophys Acta 1812:1359–1370
Merritt TJ, Sezgin E, Zhu CT, Eanes WF (2006) Triglyceride pools, flight and activity variation at the gpdh locus in drosophila melanogaster. Genetics 172:293–304
Miller FJ, Rosenfeldt FL, Zhang C et al (2003) Precise determination of mitochondrial DNA copy number in human skeletal and cardiac muscle by a pcr-based assay: lack of change of copy number with age. Nucleic Acids Res 31:e61
Miller KN, Burhans MS, Clark JP et al (2017) Aging and caloric restriction impact adipose tissue, adiponectin, and circulating lipids. Aging Cell 16(3):497–507
Monaghan P (2014) Organismal stress, telomeres and life histories. J Exp Biol 217:57–66
Neustadt J, Pieczenik SR (2008) Organ reserve and healthy aging. Integr Med 7:50–52
Noblitt SD, Huehls AM, Morris DL Jr (2007) The role of metal ion binding in generating 8-hydroxy-2′-deoxyguanosine from the nucleoside 2′-deoxyguanosine and the nucleotide 2′-deoxyguanosine-5′-monophosphate. J Inorg Biochem 101:536–542
Peleg S, Feller C, Forne I et al (2016) Life span extension by targeting a link between metabolism and histone acetylation in drosophila. EMBO Rep 17:455–469
Pesce V, Cormio A, Fracasso F et al (2001) Age-related mitochondrial genotypic and phenotypic alterations in human skeletal muscle. Free Radic Biol Med 30:1223–1233
Rattan SI (1995) Gerontogenes: real or virtual? FASEB J 9:284–286
Rattan SI (1998) The nature of gerontogenes and vitagenes. Antiaging effects of repeated heat shock on human fibroblasts. Ann N Y Acad Sci 854:54–60
Rattan SI (2014) Molecular gerontology: from homeodynamics to hormesis. Curr Pharm Des 20:3036–3039
Rattan SI (2015) Biology of ageing: principles, challenges and perspectives. Rom J Morphol Embryol 56:1251–1253
Rea S, Johnson TE (2003) A metabolic model for life span determination in caenorhabditis elegans. Dev Cell 5:197–203
Rossiello F, Herbig U, Longhese MP et al (2014) Irreparable telomeric DNA damage and persistent ddr signalling as a shared causative mechanism of cellular senescence and ageing. Curr Opin Genet Dev 26:89–95
Saito K, Tai H, Hemmi H et al (2012) Interaction between the heme and a g-quartet in a heme-DNA complex. Inorg Chem 51:8168–8176
Salvador A, Savageau MA (2003) Quantitative evolutionary design of glucose 6-phosphate dehydrogenase expression in human erythrocytes. Proc Natl Acad Sci USA 100:14463–14468
Sanders SP, Zweier JL, Kuppusamy P et al (1993) Hyperoxic sheep pulmonary microvascular endothelial cells generate free radicals via mitochondrial electron transport. J Clin Invest 91:46–52
Sansbury BE, Jones SP, Riggs DW et al (2011) Bioenergetic function in cardiovascular cells: the importance of the reserve capacity and its biological regulation. Chem Biol Interact 191:288–295
Sarkisian CA, Gruenewald TL, John Boscardin W, Seeman TE (2008) Preliminary evidence for subdimensions of geriatric frailty: the macarthur study of successful aging. J Am Geriatr Soc 56:2292–2297
Savji N, Rockman CB, Skolnick AH et al (2013) Association between advanced age and vascular disease in different arterial territories: a population database of over 3.6 million subjects. J Am Coll Cardiol 61:1736–1743
Schwerzmann K, Hoppeler H, Kayar SR, Weibel ER (1989) Oxidative capacity of muscle and mitochondria: correlation of physiological, biochemical, and morphometric characteristics. Proc Natl Acad Sci USA 86:1583–1587
Sehl ME, Yates FE (2001) Kinetics of human aging: i. Rates of senescence between ages 30 and 70 years in healthy people. J Gerontol A Biol Sci Med Sci 56:B198–B208
Shay JW, Wright WE (2005) Senescence and immortalization: role of telomeres and telomerase. Carcinogenesis 26:867–874
Shiva S, Brookes PS, Patel RP et al (2001) Nitric oxide partitioning into mitochondrial membranes and the control of respiration at cytochrome c oxidase. Proc Natl Acad Sci USA 98:7212–7217
Sloan CD, Shen L, West JD et al (2010) Genetic pathway-based hierarchical clustering analysis of older adults with cognitive complaints and amnestic mild cognitive impairment using clinical and neuroimaging phenotypes. Am J Med Genet B Neuropsychiatr Genet 153B:1060–1069
Sobenin IA, Mitrofanov KY, Zhelankin AV et al (2014) Quantitative assessment of heteroplasmy of mitochondrial genome: perspectives in diagnostics and methodological pitfalls. Biomed Res Int 2014:292017
Sternberg SA, Wershof Schwartz A, Karunananthan S et al (2011) The identification of frailty: a systematic literature review. J Am Geriatr Soc 59:2129–2138
Taormina G, Mirisola MG (2014) Calorie restriction in mammals and simple model organisms. Biomed Res Int 2014:308690
Tchkonia T, Zhu Y, Van Deursen J et al (2013) Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Investig 123:966–972
Thorburn DR, Kuchel PW (1985) Regulation of the human-erythrocyte hexose-monophosphate shunt under conditions of oxidative stress. A study using nmr spectroscopy, a kinetic isotope effect, a reconstituted system and computer simulation. Eur J Biochem 150:371–386
Ungvari Z, Parrado-Fernandez C, Csiszar A, De Cabo R (2008) Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res 102:519–528
Veltri KL, Espiritu M, Singh G (1990) Distinct genomic copy number in mitochondria of different mammalian organs. J Cell Physiol 143:160–164
Villani G, Attardi G (1997) In vivo control of respiration by cytochrome c oxidase in wild-type and mitochondrial DNA mutation-carrying human cells. Proc Natl Acad Sci USA 94:1166–1171
Watanabe S, Kawamoto S, Ohtani N, Hara E (2017) Impact of senescence-associated secretory phenotype and its potential as a therapeutic target for senescence-associated diseases. Cancer Sci 108:563–569
Wiesner RJ, Ruegg JC, Morano I (1992) Counting target molecules by exponential polymerase chain reaction: copy number of mitochondrial DNA in rat tissues. Biochem Biophys Res Commun 183:553–559
Wyckelsma VL, Mckenna MJ (2016) Effects of age on na(+), k(+)-atpase expression in human and rodent skeletal muscle. Front Physiol 7:316
Xin MG, Zhang J, Block ER, Patel JM (2003) Senescence-enhanced oxidative stress is associated with deficiency of mitochondrial cytochrome c oxidase in vascular endothelial cells. Mech Ageing Dev 124:911–919
Yadava N, Nicholls DG (2007) Spare respiratory capacity rather than oxidative stress regulates glutamate excitotoxicity after partial respiratory inhibition of mitochondrial complex i with rotenone. J Neurosci 27:7310–7317
Zimniak P (2012) What is the proximal cause of aging? Front Genet 3:189
Acknowledgement
This project was supported by the National Institute of Aging of NIH (R15AG041414), American Federation for Aging Research (AFAR), and the Ames Foundation to HA. We are thankful to Ms. Marisa Luna and Ms. Gloria Arredondo at Arrowhead Regional Medical Center (ARMC) library for their unconditioned support to this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Rights and permissions
About this article
Cite this article
Atamna, H., Tenore, A., Lui, F. et al. Organ reserve, excess metabolic capacity, and aging. Biogerontology 19, 171–184 (2018). https://doi.org/10.1007/s10522-018-9746-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-018-9746-8