Abstract
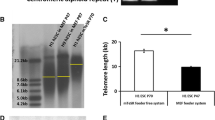
It has been widely accepted that telomere shortening acts as a cell division counting mechanism that beyond a set critical length signals cells to enter replicative senescence. In this study, we demonstrate that by simply lowering the oxygen content of the cell culture environment 10-fold (20–2%) extends the replicative lifespan of fetal bovine fibroblasts at least five-times (30–150 days). Although, low oxygen fibroblasts display a slightly slower rate (P > 0.05) of telomere attrition than their high oxygen counterparts (171 bp versus 182 bp/PD), late passage fibroblasts (>50 PD) that have extended their replicative capacity under low oxygen conditions exhibited significantly (P < 0.05) shorter telomere lengths (11,135 ± 467 bp) compared to senescent cells (25–34 PD) cultured under high oxygen conditions (14,827 ± 1173 bp). There was a significant increase (P < 0.05) in chromosomal abnormalities with continual cell division under both high and low oxygen environments, however, fibroblasts displayed a significant reduction (P < 0.001) in chromosomal abnormalities at low oxygen tensions compared to those under 20% oxygen. These apparent protective effects on telomere shortening, delayed senescence and reduced chromosomal aberrations may be attributed to the up-regulation of telomerase activity observed for fibroblasts cultured under low oxygen. These results are consistent with the idea that a critically short telomere length may not be the sole trigger of replicative senescence, but may be regulated by the integrity of telomere structure itself and/or the amount of oxidative DNA damage in the cell.






Similar content being viewed by others
Abbreviations
- FF:
-
Fetal fibroblast
- PDL:
-
Population doubling level
- ROS:
-
Reactive oxygen species
- TERT:
-
Telomerase reverse transcriptase
- TRF:
-
Terminal Restriction Fragment
References
Allsopp RC, Chang E, Kashefi-Aazam M, Rogaev EI, Piatyszek MA, Shay JW, Harley CB (1995) Telomere shortening is associated with cell division in vitro and in vivo. Exp Cell Res 220(1):194–200
Ames BN, Shigenaga MK (1992) Oxidants are a major contributor to aging. Ann N Y Acad Sci 663:85–96
Bakkenist CJ, Drissi R, Wu J, Kastan MB, Dome JS (2004) Disappearance of the telomere dysfunction-induced stress response in fully senescent cells. Cancer Res 64(11):3748–3752
Balin AK, Goodman DB, Rasmussen H, Cristofalo VJ (1977) The effect of oxygen and vitamin E on the lifespan of human diploid cells in vitro. J Cell Biol 74(1):58–67
Ben-Porath I, Weinberg RA (2004) When cells get stressed: an integrative view of cellular senescence. J Clin Invest 113(1):8–13
Blackburn EH, Chan S, Chang J, Fulton TB, Krauskopf A, McEachern M, Prescott J, Roy J, Smith C, Wang H (2000) Molecular manifestations and molecular determinants of telomere capping. Cold Spring Harb symp quant biol 65:253–263
Blanco R, Munoz P, Flores JM, Klatt P, Blasco MA (2007) Telomerase abrogation dramatically accelerates TRF2-induced epithelial carcinogenesis. Genes Dev 21(2):206–220
Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE (1998) Extension of life-span by introduction of telomerase into normal human cells. Science 279(5349):349–352
Bond JA, Wyllie FS, Wynford-Thomas D (1994) Escape from senescence in human diploid fibroblasts induced directly by mutant p53. Oncogene 9(7):1885–1889
Brown JP, Wei W, Sedivy JM (1997) Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts. Science 277(5327):831–834
Chen Q, Fischer A, Reagan JD, Yan LJ, Ames BN (1995) Oxidative DNA damage and senescence of human diploid fibroblast cells. Proc Natl Acad Sci USA 92(10):4337–4341
Collins K (2000) Mammalian telomeres and telomerase. Curr Opin Cell Biol 12(3):378–383
Counter CM, Hirte HW, Bacchetti S, Harley CB (1994) Telomerase activity in human ovarian carcinoma. Proc Natl Acad Sci USA 91(8):2900–2904
Cristofalo VJ, Allen RG, Pignolo RJ, Martin BG, Beck JC (1998) Relationship between donor age and the replicative lifespan of human cells in culture: a reevaluation. Proc Natl Acad Sci USA 95(18):10614–10619
Cristofalo VJ, Beck J, Allen RG (2003) Cell senescence: an evaluation of replicative senescence in culture as a model for cell aging in situ. J Gerontol 58(9):B776–B779; discussion 779–781
d’Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP (2003) A DNA damage checkpoint response in telomere-initiated senescence. Nature 426(6963):194–198
Davis T, Skinner JW, Faragher RG, Jones CJ, Kipling D (2005) Replicative senescence in sheep fibroblasts is a p53 dependent process. Exp Gerontol 40(1–2):17–26
Duan J, Duan J, Zhang Z, Tong T (2005) Irreversible cellular senescence induced by prolonged exposure to H2O2 involves DNA-damage-and-repair genes and telomere shortening. Int J Biochem Cell Biol 37(7):1407–1420
Favetta LA, Robert C, King WA, Betts DH (2004) Expression profiles of p53 and p66shc during oxidative stress-induced senescence in fetal bovine fibroblasts. Exp Cell Res 299(1):36–48
Favetta LA, St John EJ, King WA, Betts DH (2007) High levels of p66shc and intracellular ROS in permanently arrested early embryos. Free Radic Biol Med 42(8):1201–1210
Figueroa R, Lindenmaier H, Hergenhahn M, Nielsen KV, Boukamp P (2000) Telomere erosion varies during in vitro aging of normal human fibroblasts from young and adult donors. Cancer Res 60(11):2770–2774
Frenck RW Jr., Blackburn EH, Shannon KM (1998) The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci USA 95(10):5607–5610
Golczewski JA (1984) Effect of 2-mercaptoethylamine on proliferation and lifespan of WI-38 cells. Exp Gerontol 19(1):7–11
Goldstein S (1974) Aging in vitro. Growth of cultured cells from the Galapagos tortoise. Exp Cell Res 83:297–302
Goldstein S, Moerman EJ, Soeldner JS, Gleason RE, Barnett DM (1978) Chronologic and physiologic age affect replicative life-span of fibroblasts from diabetic, prediabetic, and normal donors. Science 199(4330):781–782
Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, de Lange T (1999) Mammalian telomeres end in a large duplex loop. Cell 97(4):503–514
Haendeler J, Hoffmann J, Diehl JF, Vasa M, Spyridopoulos I, Zeiher AM, Dimmeler S (2004) Antioxidants inhibit nuclear export of telomerase reverse transcriptase and delay replicative senescence of endothelial cells. Circ Res 94(6):768–775
Harley CB, Futcher AB, Greider CW (1990) Telomeres shorten during ageing of human fibroblasts. Nature 345(6274):458–460
Hastie ND, Dempster M, Dunlop MG, Thompson AM, Green DK, Allshire RC (1990) Telomere reduction in human colorectal carcinoma and with ageing. Nature 346(6287):866–868
Hayflick L (1965) the limited in vitro lifetime of human diploid cell strains. Exp Cell Res 37:614–636
Hayflick L, Moorhead PS (1961) The serial cultivation of human diploid cell strains. Exp Cell Res 25:585–621
Hecht A, Strahl-Bolsinger S, Grunstein M (1996) Spreading of transcriptional repressor SIR3 from telomeric heterochromatin. Nature 383(6595):92–96
Holmes GE, Bernstein C, Bernstein H (1992) Oxidative and other DNA damages as the basis of aging: a review. Mutat Res 275(3–6):305–315
Holt SE, Wright WE, Shay JW (1997) Multiple pathways for the regulation of telomerase activity. Eur J Cancer 33(5):761–766
Honda S, Matsuo M (1983) Shortening of the in vitro lifespan of human diploid fibroblasts exposed to hyperbaric oxygen. Exp Gerontol 18(5):339–345
Hornsby PJ, Harris SE (1987) Oxidative damage to DNA and replicative lifespan in cultured adrenocortical cells. Exp Cell Res 168(1):203–217
Huffman KE, Levene SD, Tesmer VM, Shay JW, Wright WE (2000) Telomere shortening is proportional to the size of the G-rich telomeric 3′-overhang. J Biol Chem 275(26):19719–19722
Hyeon Joo O, Hande MP, Lansdorp PM, Natarajan AT (1998) Induction of telomerase activity and chromosome aberrations in human tumour cell lines following X-irradiation. Mutat Res 401(1–2):121–131
Jones CJ, Kipling D, Morris M, Hepburn P, Skinner J, Bounacer A, Wyllie FS, Ivan M, Bartek J, Wynford-Thomas D, Bond JA (2000) Evidence for a telomere-independent “clock” limiting RAS oncogene-driven proliferation of human thyroid epithelial cells. Mol Cell Biol 20(15):5690–5699
Karlseder J, Smogorzewska A, de Lange T (2002) Senescence induced by altered telomere state, not telomere loss. Science 295(5564):2446–2449
Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW (1994) Specific association of human telomerase activity with immortal cells and cancer. Science 266(5193):2011–2015
Kim NW, Wu F (1997) Advances in quantification and characterization of telomerase activity by the telomeric repeat amplification protocol (TRAP). Nucleic Acids Res 25(13):2595–2597
Kurz DJ, Decary S, Hong Y, Trivier E, Akhmedov A, Erusalimsky JD (2004) Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J Cell Sci 117(Pt 11):2417–2426
Leteurtre F, Li X, Gluckman E, Carosella ED (1997) Telomerase activity during the cell cycle and in gamma-irradiated hematopoietic cells. Leukemia 11(10):1681–1689
Li GZ, Eller MS, Firoozabadi R, Gilchrest BA (2003) Evidence that exposure of the telomere 3’ overhang sequence induces senescence. Proc Natl Acad Sci USA 100(2):527–531
Lindsey J, McGill NI, Lindsey LA, Green DK, Cooke HJ (1991) In vivo loss of telomeric repeats with age in humans. Mutat Res 256(1):45–48
Marcand S, Buck SW, Moretti P, Gilson E, Shore D (1996) Silencing of genes at nontelomeric sites in yeast is controlled by sequestration of silencing factors at telomeres by Rap 1 protein. Genes Dev 10(11):1297–1309
Martens UM, Chavez EA, Poon SS, Schmoor C, Lansdorp PM (2000) Accumulation of short telomeres in human fibroblasts prior to replicative senescence. Exp Cell Res 256(1):291–299
Martin SG, Laroche T, Suka N, Grunstein M, Gasser SM (1999) Relocalization of telomeric Ku and SIR proteins in response to DNA strand breaks in yeast. Cell 97(5):621–633
Mastromonaco GF, Perrault SD, Betts DH, King WA (2006) Role of chromosome stability and telomere length in the production of viable cell lines for somatic cell nuclear transfer. BMC Dev Biol 6(1):41
Masutomi K, Yu EY, Khurts S, Ben-Porath I, Currier JL, Metz GB, Brooks MW, Kaneko S, Murakami S, DeCaprio JA, Weinberg RA, Stewart SA, Hahn WC (2003) Telomerase maintains telomere structure in normal human cells. Cell 114(2):241–253
Meier A, Fiegler H, Munoz P, Ellis P, Rigler D, Langford C, Blasco MA, Carter N, Jackson SP (2007) Spreading of mammalian DNA-damage response factors studied by ChIP-chip at damaged telomeres. EMBO J 26(11):2707–2718
Morin GB (1989) The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell 59(3):521–529
Moyzis RK, Buckingham JM, Cram LS, Dani M, Deaven LL, Jones MD, Meyne J, Ratliff RL, Wu JR (1988) A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci USA 85(18):6622–6626
Nishi H, Nakada T, Kyo S, Inoue M, Shay JW, Isaka K (2004) Hypoxia-inducible factor 1 mediates upregulation of telomerase (hTERT). Mol Cell Biol 24(13):6076–6083
Noda A, Ning Y, Venable SF, Pereira-Smith OM, Smith JR (1994) Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res 211(1):90–98
Olovnikov AM (1973) A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol 41(1):181–190
Ouellette MM, Liao M, Herbert BS, Johnson M, Holt SE, Liss HS, Shay JW, Wright WE (2000) Subsenescent telomere lengths in fibroblasts immortalized by limiting amounts of telomerase. J Biol Chem 275(14):10072–10076
Packer L, Fuehr K (1977) Low oxygen concentration extends the lifespan of cultured human diploid cells. Nature 267(5610):423–425
Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J (2003) Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat Cell Biol 5(8):741–747
Passos JF, Saretzki G, Ahmed S, Nelson G, Richter T, Peters H, Wappler I, Birket MJ, Harold G, Schaeuble K, Birch-Machin MA, Kirkwood TB, von Zglinicki T (2007) Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol 5(5):e110
Petersen S, Saretzki G, von Zglinicki T (1998) Preferential accumulation of single-stranded regions in telomeres of human fibroblasts. Exp Cell Res 239(1):152–160
Rohme D (1981) Ageing and the fusion sensitivity potential of human cells in culture: relation to tissue origin, donor age, and in vitro culture level and condition. Mech Ageing Dev 16(3):241–253
Saito H, Hammond AT, Moses RE (1995) The effect of low oxygen tension on the in vitro-replicative life span of human diploid fibroblast cells and their transformed derivatives. Exp Cell Res 217(2):272–279
Saretzki G, Sitte N, Merkel U, Wurm RE, von Zglinicki T (1999) Telomere shortening triggers a p53-dependent cell cycle arrest via accumulation of G-rich single stranded DNA fragments. Oncogene 18(37):5148–5158
Seimiya H, Tanji M, Oh-hara T, Tomida A, Naasani I, Tsuruo T (1999) Hypoxia up-regulates telomerase activity via mitogen-activated protein kinase signaling in human solid tumor cells. Biochem Biophys Res Commun 260(2):365–370
Serrano M, Hannon GJ, Beach D (1993) A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature 366(6456):704–707
Shay JW, Pereira-Smith OM, Wright WE (1991) A role for both RB and p53 in the regulation of human cellular senescence. Exp Cell Res 196(1):33–39
Shay JW, Wright WE, Brasiskyte D, Van der Haegen BA (1993) E6 of human papillomavirus type 16 can overcome the M1 stage of immortalization in human mammary epithelial cells but not in human fibroblasts. Oncogene 8(6):1407–1413
Smith JR, Venable S, Roberts TW, Metter EJ, Monticone R, Schneider EL (2002) Relationship between in vivo age and in vitro aging: assessment of 669 cell cultures derived from members of the Baltimore longitudinal study of aging. J Gerontol 57(6):B239–B246
Smogorzewska A, van Steensel B, Bianchi A, Oelmann S, Schaefer MR, Schnapp G, de Lange T (2000) Control of human telomere length by TRF1 and TRF2. Mol Cell Biol 20(5):1659–1668
Sohal RS, Allen RG (1990) Oxidative stress as a causal factor in differentiation and aging: a unifying hypothesis. Exp Gerontol 25(6):499–522
Sokal RR, Rohlf FJ (1981) Biometry: the principles and practice of statistics in biological research. W. H. Freeman, San Francisco, pp xviii, 859 p
Steinert S, Shay JW, Wright WE (2000) Transient expression of human telomerase extends the life span of normal human fibroblasts. Biochem Biophys Res Commun 273(3):1095–1098
Stewart SA, Ben-Porath I, Carey VJ, O’Connor BF, Hahn WC, Weinberg RA (2003) Erosion of the telomeric single-strand overhang at replicative senescence. Nat Genet 33(4):492–496
te Poele RH, Okorokov AL, Jardine L, Cummings J, Joel SP (2002) DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res 62(6):1876–1883
Therkelsen AJ, Nielsen A, Koch J, Hindkjaer J, Kolvraa S (1995) Staining of human telomeres with primed in situ labeling (PRINS). Cytogenet Cell Genet 68(1–2):115–118
van Steensel B, Smogorzewska A, de Lange T (1998) TRF2 protects human telomeres from end-to-end fusions. Cell 92(3):401–413
Vaziri H, Benchimol S (1998) Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr Biol 8(5):279–282
von Zglinicki T, Pilger R, Sitte N (2000) Accumulation of single-strand breaks is the major cause of telomere shortening in human fibroblasts. Free Radic Biol Med 28(1):64–74
von Zglinicki T, Saretzki G, Docke W, Lotze C (1995) Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp Cell Res 220(1):186–193
Yu GL, Bradley JD, Attardi LD, Blackburn EH (1990) In vivo alteration of telomere sequences and senescence caused by mutated Tetrahymena telomerase RNAs. Nature 344(6262):126–132
Zhu J, Wang H, Bishop JM, Blackburn EH (1999) Telomerase extends the lifespan of virus-transformed human cells without net telomere lengthening. Proc Natl Acad Sci USA 96(7):3723–3728
Acknowledgements
The Barbara Graham Memorial Trust, Natural Sciences and Engineering Research Council (NSERC) of Canada, Canadian Institutes of Health Research (CIHR), and the Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA) supported this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Betts, D.H., Perrault, S.D. & King, W.A. Low oxygen delays fibroblast senescence despite shorter telomeres. Biogerontology 9, 19–31 (2008). https://doi.org/10.1007/s10522-007-9113-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-007-9113-7