Abstract
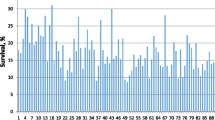
A selective breeding program was established to improve the growth and survival of the cultured giant freshwater prawn Macrobrachium rosenbergii. The response to selection was estimated for the survival of M. rosenbergii using a fully pedigreed synthetic population formed by three introduced strains. The data included 122,761 progeny from 437 sires and 723 dams in seven generations with a nested mating structure. The genetic parameters and estimated breeding values (EBVs) were estimated using a generalized linear mixed model with the probit link function. The realized response was estimated from the difference in the marginal means of survival for the selection and control populations, while the predicted response was obtained from the difference in the mean retransformed survival rate based on the survival EBVs between generations. The realized genetic gain in survival from the G1 to G6 generation ranged from −1.24 to 2.72 %. The accumulated realized genetic gain (5.02 %) expressed as a percentage was 8.46 %. Across the generations, high heritability (0.401 ± 0.020, Set 1) was obtained when using the model without the c effect and was significantly different from zero (P < 0.05). However, the low heritability and common environment (0.013 ± 0.011 and 0.088 ± 0.007, Set 2) were estimated using the model that included the c effect. The accumulated predicted gains (6.29 and 0.61 %, respectively) from the Set 1 and Set 2 parameters over the five generations of selection expressed as proportions were 9.08 and 0.87 %, respectively. The low genetic gain for survival is most likely caused by a low relative weight in the selection index and reduced genetic variation because of consecutive between-family selection.

Similar content being viewed by others
Abbreviations
- EBV:
-
Estimated breeding value
- VIE:
-
Visible implant elastomer
- BLUP:
-
Best linear unbiased prediction
- C:
-
The common environmental effect
References
Aguilar I, Misztal I, Johnson DL, Legarra A, Tsuruta S, Lawlor TJ (2010) Hot topic: a unified approach to utilize phenotypic, full pedigree, and genomic information for genetic evaluation of Holstein final score. J Dairy Sci 93:743–752
Argue BJ, Arce SM, Lotz JM, Moss SM (2002) Selective breeding of Pacific white shrimp (Litopenaeus vannamei) for growth and resistance to Taura Syndrome Virus. Aquaculture 204:447–460
Castillo-Juárez H, Casares JCQ, Campos-Montes G, Villela CC, Ortega AM, Montaldo HH (2007) Heritability for body weight at harvest size in the Pacific white shrimp, Penaeus (Litopenaeus) vannamei, from a multi-environment experiment using univariate and multivariate animal models. Aquaculture 273:42–49
Cuéllar-Anjel J, White-Noble B, Schofield P, Chamorro R, Lightner DV (2012) Report of significant WSSV-resistance in the Pacific white shrimp, Litopenaeus vannamei, from a Panamanian breeding program. Aquaculture 368–369:36–39
Dégremont L, Bédier E, Boudry P (2010) Summer mortality of hatchery-produced Pacific oyster spat (Crassostrea gigas). II. Response to selection for survival and its influence on growth and yield. Aquaculture 299:21–29
FAO (2013) FishStatJ, a tool for fishery statistics analysis Release: 2.0.0. http://www.fao.org/fishery/statistics/software/fishstatj/en
Fishback AG, Danzmann RG, Ferguson MM, Gibson JP (2002) Estimates of genetic parameters and genotype by environment interactions for growth traits of rainbow trout (Oncorhynchus mykiss) as inferred using molecular pedigrees. Aquaculture 206:137–150
Gilmour A, Cullis B, Welham S, Gogel B, Thompson R (2004) An efficient computing strategy for prediction in mixed linear models. Comput Stat Data An 44:571–586
Gilmour AR, Gogel BJ, Cullis BR, Thompson R (2009) ASReml User Guide Release 3.0. VSN International Ltd, Hemel Hempstead
Gitterle T, Salte R, Gjerde B, Cock J, Johansen H, Salazar M, Lozano C, Rye M (2005a) Genetic (co)variation in resistance to White Spot Syndrome Virus (WSSV) and harvest weight in Penaeus (Litopenaeus) vannamei. Aquaculture 246:139–149
Gitterle T, Rye M, Salte R, Cock J, Johansen H, Lozano C, Suárez JA, Gjerde B (2005b) Genetic (co)variation in harvest body weight and survival in Penaeus (Litopenaeus) vannamei under standard commercial conditions. Aquaculture 243:83–92
Gitterle T, Ødegård J, Gjerde B, Rye M, Salte R (2006) Genetic parameters and accuracy of selection for resistance to White Spot Syndrome Virus (WSSV) in Penaeus (Litopenaeus) vannamei using different statistical models. Aquaculture 251:210–218
Gitterle T, Johansen H, Erazo C, Lozano C, Cock J, Salazar M, Rye M (2007) Response to multi-trait selection for harvest weight, overall survival, and resistance to white spot syndrome virus (WSSV) in Penaeus (Litopenaeus) vannamei. Aquaculture 272:S262
Gjedrem T, Olesen I (2005) Basic statistical parameters. In: Gjedrem T (ed) Selection and breeding programs in aquaculture,1rd edn. Springer, Dordrecht
Gjedrem T, Robinson N, Rye M (2012) The importance of selective breeding in aquaculture to meet future demands for animal protein: a review. Aquaculture 350–353:117–129
Huang YC, Yin ZX, Weng SP, He JG, Li SD (2012) Selective breeding and preliminary commercial performance of Penaeus vannamei for resistance to white spot syndrome virus (WSSV). Aquaculture 364–365:111–117
Kenway M, Macbeth M, Salmon M, McPhee C, Benzie J, Wilson K, Knibb W (2006) Heritability and genetic correlations of growth and survival in black tiger prawn Penaeus monodon reared in tanks. Aquaculture 259:138–145
Krishna G, Gopikrishna G, Gopal C, Jahageerdar S, Ravichandran P, Kannappan S, Pillai SM, Paulpandi S, Kiran RP, Saraswati R, Venugopal G, Kumar D, Gitterle T, Lozano C, Rye M, Hayes B (2011) Genetic parameters for growth and survival in Penaeus monodon cultured in India. Aquaculture 318:74–78
Luan S, Yang GL, Wang JY, Luo K, Zhang YF, Gao Q, Hu HL, Kong J (2012) Genetic parameters and response to selection for harvest body weight of the giant freshwater prawn Macrobrachium rosenbergii. Aquaculture 362–363:88–96
Luo MF, Boettcher PJ, Schaeffer LR, Dekkers J (2001) Bayesian inference for categorical traits with an application to variance component estimation. J Dairy Sci 84:694–704
Martìnez V, Neira R, Gall GAE (1999) Estimation of genetic parameters from pedigreed populations: lessons from analysis of alevin weight in Coho salmon (Oncorhynchus kisutch). Aquaculture 180:223–236
Moss SM, Doyle RW (2005) Lightner DV (2005) Breeding shrimp for disease resistance: challenges and opportunities for improvement. In: Lester R (ed) Walker P. Manila, Diseases in Asian Aquaculture
Nguyen NH, Khaw HL, Ponzoni RW, Hamzah A, Kamaruzzaman N (2007) Can sexual dimorphism and body shape be altered in Nile tilapia (Oreochromis niloticus) by genetic means? Aquaculture 272:S38–S46
Ødegård J, Meuwissen TH, Heringstad B, Madsen P (2010) A simple algorithm to estimate genetic variance in an animal threshold model using Bayesian inference. Genet Sel Evol 42:29–35
Ødegård J, Baranski M, Gjerde B, Gjedrem T (2011) Methodology for genetic evaluation of disease resistance in aquaculture species: challenges and future prospects. Aquac Res 42:103–114
Pante MJR, Gjerde B, McMillan I, Misztal I (2002) Estimation of additive and dominance genetic variances for body weight at harvest in rainbow trout, Oncorhynchus mykiss. Aquaculture 204:383–392
Rutten MJM, Komen H, Bovenhuis H (2005) Longitudinal genetic analysis of Nile tilapia (Oreochromis niloticus L.) body weight using a random regression model. Aquaculture 246:101–113
Rye M, Lillevik KM, Gjerde B (1990) Survival in early life of Atlantic salmon and rainbow trout: estimates of heritabilities and genetic correlations. Aquaculture 89:209–216
Shaw FH, Shaw RG, Wilkinson GS, Turelli M (1995) Changes in genetic variances and covariances: G whiz! Evolution 49:1260–1267
Vehviläinen H, Kause A, Quinton C, Koskinen H, Paananen T (2008) Survival of the currently fittest: genetics of rainbow trout survival across time and space. Genetics 180:507–516
Wahab MA, Ahmad Al Nahid S, Ahmed N, Haque MM, Karim M (2012) Current status and prospects of farming the giant river prawn Macrobrachium rosenbergii (De Man) in Bangladesh. Aquac Res 43:970–983
Welham S, Cullis B, Gogel B, Gilmour A, Thompson R (2004) Prediction in linear mixed models. Aust Nz J Stat 46:325–347
Acknowledgments
This work was supported by grants from the Special Fund for Agro-scientific Research in the Public Interest (200903045), and the National Key Technology R&D Program (2012BAD26B04). The authors would also like to thank Drs Arthur Richard Gilmour (VSN International), Bjarne Gjerde (Nofima), Raul W. Ponzoni (The WorldFish Center), Jørgen Ødegård (Nofima), and Yuxi Zhang (Qingdao Agriculture University) for their constructive suggestions in the data analysis and revision of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Sheng Luan and Guoliang Yang have contributed equally to the work.
Rights and permissions
About this article
Cite this article
Luan, S., Yang, G., Wang, J. et al. Selection responses in survival of Macrobrachium rosenbergii after performing five generations of multi-trait selection for growth and survival. Aquacult Int 22, 993–1007 (2014). https://doi.org/10.1007/s10499-013-9722-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-013-9722-x