Abstract
Pikeperch, Sander lucioperca (L.), has been identified as one of the most perspective candidates for diversification of freshwater aquaculture. However, some aspects of production are still being developed, and controlled reproduction is one of the bottlenecks. The aim of the present study was to compare the effectiveness of different commercial spawning agents in the induction of final oocyte maturation (FOM) and ovulation in wild spawners. Within the study, four spawning agents [human chorionic gonadotropin (hCG), mixed human and horse gonadotropin (PG-600), carp pituitary (CPH) and mammalian GnRH analogue combined with metoclopramide (Ovopel)] in different thermal regimes (13 and 15 °C) were tested. In both thermal regimes, the highest (P < 0.05) ovulation rate among the treatment groups was obtained after stimulation with hCG (100 % in both cases). Latency time was the shortest in groups where CPH was used (2–3 and 3–4 days for 15 and 13 °C) and was similar in the remaining groups (3–4 and 4–5 days for 15 and 13 °C, respectively). Embryo survival was the highest in groups treated with hCG (78.9 and 81.3 % at hatching stage for 15 and 13 °C, respectively). Hormonal stimulation did not significantly affect spermiation rate or spermatozoa motility (P > 0.05). Based on the obtained results, hCG can be recommended for induction of FOM and ovulation in pikeperch. In addition, the thermal regime within the tested range seemed to have no effect on the reproduction outcome, and the application of lower temperature only prolonged the time of ovulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pikeperch, Sander lucioperca (L.), is a species of great economic importance on the European market. Therefore, in recent years it has become an important species for diversification of freshwater aquaculture (Kestemont and Melard 2000; Philipsen 2008; Wang et al. 2009a). This has necessitated developing procedures for intensive culture in closed recirculating systems (Kestemont et al. 2007; Philipsen 2008). Artificial reproduction directly affects the effectiveness of further culture procedures and is one of the most important stages in intensive aquacultural production (Kucharczyk et al. 2007; Muller-Bellecke and Zienert 2008; Wang et al. 2009b; Zakęś and Demska-Zakęś 2009).
Reproduction of freshwater fish from wild populations is often impossible without hormonal stimulation (Krejszeff et al. 2009, 2010a). This does not apply to percids, in which ovulation has been observed without the need to use hormonal preparations (Kucharczyk et al. 1996, 1998, 2007; Ronyai and Lengyel 2010). However, in that case, it is difficult to predict the moment of ovulation (Kucharczyk et al. 2007; Szczerbowski et al. 2009; Żarski et al. 2011b, 2012), which greatly hinders obtaining eggs and their controlled fertilisation, and even makes it impossible, because pikeperch as well as Eurasian perch, Perca fluviatilis L., spontaneously release eggs in a tank (Ronyai and Lengyel 2010; Żarski et al. 2011b, 2012). Thus, hormonal stimulation is used in artificial reproduction of the species (Kucharczyk et al. 2007; Zakęś and Demska-Zakęś 2009), which induces final oocyte maturation (FOM) and ovulation. Therefore, it is very important to verify the latency time for these species due to the application of different reproduction procedures, including the use of hormonal preparations.
Final oocyte maturation is stimulated by two types of hormonal preparations, which affected to endocrinological response on two different levels. Preparations which contain gonadotropin-releasing hormone (GnRH) analogues affect the pituitary gland to excretion of endogenous gonadotropins (GtH), which stimulate FOM. Preparations such as carp pituitary homogenate (CPH) and human chorionic gonadotropin (hCG) affect fish gonads directly, thus stimulating FOM (Zohar and Mylonas 2001; Yaron et al. 2009). Different mechanisms of action of different preparations result in different latency times and different effectiveness, which affects egg quality (Żarski et al. 2009; Targońska et al. 2010; Bobe and Labbe 2010) which, in turn, may influence the effectiveness of production. Moreover, different prices of the preparations may significantly affect the cost-effectiveness of the production process (Hakuć-Błażowska et al. 2009, 2010). To date, only a limited amount of data has been published which could be used to compare the effectiveness of different hormonal preparations at the same time in the same population (or stock) of pikeperch during the artificial reproduction (Kucharczyk et al. 2007).
The temperature of water before and after hormonal stimulation is another very important element of the artificial reproduction procedure. A low temperature may result in prolonging latency time and in unsynchronisation of ovulation (Żarski et al. 2010), which could affect larvae growth differentiation and, in the case of predatory species, cannibalism intensification (Baras and Jobling 2002; Baras et al. 2003). On the other hand, a high temperature may result in a serious disturbance of FOM and a decrease in egg quality (Targońska et al. 2010). To date, no data have been published on the effect of temperature on the effectiveness of induction of ovulation in fully mature pikeperch females.
The aim of this study was to determine the effectiveness of different hormonal preparations under different thermal regimes in artificial reproduction of the pikeperch.
Materials and methods
Broodstock management and handling
Pikeperch spawners (n = 126; with average weight of 1,536 ± 359 g and average total length of 54.2 ± 5.8 cm) were caught with gillnets in Lake Wadąg (in north-eastern Poland) in early spring, when the average 24-hour temperature does not exceed 10 °C. The fish were transported in foil bags with oxygen to the hatchery of the Department of Lake and River Fisheries of the University of Warmia and Mazury in Olsztyn (Poland). Immediately after transporting, an oocyte sample (at least 30 oocytes in each sample) was taken from each female fish with a catheter (with 2 mm external and 1.2 mm internal diameter). Subsequently, the samples were fixed in Serra solution (70 % ethanol, 40 % formaldehyde and 99.5 % glacial acetic acid in proportion 6:3:1). After the cytoplasm clarification (after 2–3 min of exposure) the maturity of oocytes was determined based on the classification proposed for the pikeperch by Żarski et al. (2012). Only those female fish for which the maturation was between stage III (n = 28) and IV (n = 32) were taken for further procedures. Fish at those stages were chosen because on the basis of the published results of reproduction of pikeperch (Żarski et al. 2012) and Eurasian perch, Perca fluviatilis (Żarski et al. 2011a), at different maturational stages it could be concluded that the best and the most comparable results of induction of ovulation are to inject the fish at maturation stage III and IV of the classification proposed by Zarski et al. (2012). Subsequently, all the chosen (at the maturational stages III and IV) fish were labelled with the floy-tags and randomly divided into 10 groups. Male and female fish were kept separately in six 1,000-L tanks (2 tanks for males and 4 tanks for females were used) with controllable temperature and photoperiod (Kujawa et al. 1999). Tanks operated in a flow-through system (water flow was 5 L min−1). Initially, the temperature was set at 10 °C in all the tanks. Hormonal stimulation was performed after 24 h, followed by a gradual (<1 °C h−1) increase in the temperature by 3 or 5 °C, depending on the experimental group. The photoperiod was 12 h (12L/12D) in each experimental group. For all the manipulations, the fish were anaesthetised in MS-222 solution (150 mg L−1).
Description of hormonal agents
Four hormonal agents were used in this experiment:
-
hCG—human chorionic gonadotropin (Biomed, Poland);
-
PG-600—mixture of hCG and pregnant mare serum gonadotropin (PMSG) (proportion 1:2) (Intervet, the Netherlands);
-
CPH—carp pituitary homogenate (Argent, USA);
-
Ovopel—combination of a mammalian analogue of GnRH with metoclopramide (dopamine antagonist), available as pellets, with one pellet containing 18–20 μg of the analogue and 10 mg of metoclopramide (Agrofish, Hungary) (Horvath et al. 1997).
Gonadotropins (hCG and PG-600) were dissolved in physiological saline (0.9 % solution of NaCl). CPH and Ovopel were homogenised and subsequently diluted in physiological saline. Control groups were given injections of physiological saline (0.9 % solution of NaCl).
Experimental design
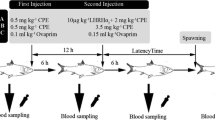
The fish in each group were given a single intraperitoneal injection of a different hormonal preparation. Subsequently, each experimental group was divided into two separate groups, with each of them exposed to a different temperature following the injection (13 or 15 °C). Such thermal regimes were described as the lower and the upper limits for pikeperch artificial reproduction (Kucharczyk et al. 2007). The procedure was identical for the fish in the control groups. This gave five groups (four experimental ones and one control) for each of the tested thermal regime. There were four male and six female fish in each of the ten groups.
The hormonal preparations were given in the following doses (according to Kucharczyk et al. 2007):
-
hCG—500 IU kg−1;
-
PG-600—500 IU kg−1;
-
CPH—4 mg kg−1;
-
Ovopel—2 pellet kg−1.
The doses of the gonadotropins (hCG and PG-600) were the average doses applied in artificial reproduction of pikeperch (between 400 and 600 IU per kg of BW; see Zakes and Demska-Zakes 2009). Carp pituitary homogenate was given in a dose which was the most effective in this species (Rónyai 2007). Ovopel was given at the commonly applied dose when it was used in a single injection (e.g. Targońska et al. 2010).
Data collection, analysis and statistics
Ovulation control in females (by delicately massaging their abdomens) was started 2 days after the injections and performed every 8 h until ovulation was detected. After eggs were taken from the first female in a particular treatment group, ovulation control in the females from this group was performed every 3–4 h (the females in each particular group were possible to recognise easily in the tank due to the different colour of the floy-tags used for each of the treatment groups). For recognition of the ovulation, females were only gently sedated in the MS-222 solution (150 mg L−1), and only after the ovulation was certified, fish were subjected to the full (surgical) anaesthesia in the same solution. Each female was checked for ovulation no more than 7 times. Eggs were collected in dry plastic containers, which were kept under cover at 10 °C until fertilisation (not longer than 30 min). Only sperm in which at least 80 % spermatozoa motility was observed was used for fertilisation. Sperm was collected to separate dry syringes by delicate abdomen massage and was determined by a subjective method under a light microscope (magnification ×400) (±10 %) (as described by Cejko et al. 2010), separately for each male, each time before the sperm was used for fertilisation. Subsequently, three egg samples from each female (300–400 eggs from each) were placed on Petri dishes and fertilised with a mixture of sperm (100 μl per sample) from at least three males from the same group as the female. The dishes were subsequently placed in 1-L glass tanks in the experimental water-recirculating system, described in detail by Krejszeff et al. (2010b). The temperature of incubation was 14 °C. Gentle water flow (0.5 L min−1) was ensured during incubation in each tank.
The following parameters were recorded and analysed statistically: ovulation and spermiation rates, latency time (between the injection and ovulation), reproductive period (between the first and the last ovulated female), spawners survival during the experiment, embryo survival rate after 72 h from fertilisation, hatching rate and spermatozoa motility. Embryo survival rate was determined based on 100 randomly chosen eggs, which were photographed under a stereoscopic microscope (Leica MZ 12.5, Germany) with ProgRes® Capture Pro 2.5 software (Jenoptic, Germany). The percentage of hatched larvae was determined on day 3 following the start of hatching.
All the results presented in this paper are expressed as arithmetic mean with standard deviation (±SD) or range in case of spermatozoa motility and latency time. All the percentage data were subjected to arc-sine transformation before being analysed statistically. The normality of the distribution of the data was checked with the Shapiro–Wilk test (if applicable). The results of spermiation rate, spermatozoa motility, ovulation rate, embryos survival, hatching rate and survival of spawners were analysed by analysis of variance (one-way ANOVA). When the analysis of variance showed statistical differences, Duncan’s post hoc test was applied (α = 0.05). Data regarding the latency time were analysed with Kruskal–Wallis test (α = 0.05). Statistical analysis was performed with Statistica 9.1 software (StatSoft Inc., USA).
Results
Hormonal stimulation was not necessary to induce spermiation, and sperm was obtained from all the males. Although sperm motility was slightly lower in the control groups as compared to the experimental groups, no statistically significant differences were found (P > 0.05). In each of the experimental group, at least one male died during the experimental period (Tables 1, 2).
The results for each group within each of the thermal regimes showed the high effectiveness of hCG, because all the females ovulated at both 13 °C (Table 1) and 15 °C (Table 2). Ovulation was found to have taken place in five females (83 %) following application of PG-600 under both thermal regimes. The application of CPH and Ovopel induced ovulation in 67 % of females. The only difference related to the ovulation rate (P < 0.05) was found in the control group as compared to the experimental groups at 13 °C, where eggs were obtained from one female. At 15 °C no female from the control group ovulated.
The highest embryo survival rate after 72 h from fertilisation was observed in the group treated with hCG (P < 0.05) under both the thermal regimes (88.3 and 84.4 % at 13 and 15 °C, respectively). Embryo survival rates in the other experimental groups within each of the thermal regimes were comparable (78.2–80.3 % and 72.3–78.3 % at 13 and 15 °C, respectively). The application of CPH at 13 °C resulted in the lowest percentage of hatched larvae (56.8 %) (P < 0.05) of all the 10 groups. The hatching rate at 15 °C was comparable to the values obtained in the group in which Ovopel was used (62.3 and 63.2 % for CPH and Ovopel, respectively). However, stimulation with Ovopel greatly reduced the percentage of hatched larvae (P < 0.05) as compared to application of PG-600 (72.6 and 70.3 % at 13 and 15 °C, respectively). The highest percentage of hatched larvae in both the thermal regimes (P < 0.05) was recorded following injections with hCG (81.3 and 78.9 % at 13 and 15 °C, respectively).
The ovulation period (between the first and the last ovulated female) in all the experimental groups was between 43 and 49 h. No significant differences were found (P > 0.05) within each of the thermal regimes with respect to the latency time between hCG, PG-600 and Ovopel. The earliest ovulation (P < 0.05) was observed at 15 °C following the application of CPH (2–3 days), whereas it took place much later (P < 0.05) following the application of hCG, PG-600 and Ovopel (4–5 days) at 13 °C. The application of CPH at 13 °C [the shortest latency time (P < 0.05) of all the experimental groups at 13 °C] and hCG, PG-600 and Ovopel at 15 °C resulted in obtaining eggs within a comparable period of time (3–4 days). The latest ovulation (P < 0.05) was observed in female in the control group (6 days). The females’ survival rate during the experiment in all the experimental groups ranged from 70 to 100 %. Two females (one in each of the thermal regime) died shortly after the eggs stripping (Tables 1, 2).
Discussion
The results of this experiment confirm previous reports on the shorter period of latency following the application of CPH as compared to Ovopel under each of the thermal regimes tested. This has been reported for the carp, Cyprinus carpio (L.) (Brzuska 2003), and the goldfish, Carassius auratus auratus (L.) (Targońska and Kucharczyk 2011). The difference probably stems from different mechanisms of action of those preparations, where GtH contained in CPH directly affects gonads and induces ovulation much faster than Ovopel, whose action involves releasing endogenous GtH (Zohar and Mylonas 2001; Yaron et al. 2009). However, the latency time following the use of Ovopel was very close to that recorded following the application of hCG and PG-600, whose action takes place on the same level as that of CPH. A similar relationship has been observed for the goldfish (Targońska and Kucharczyk 2011). In an experiment conducted by Zakęś and Demska-Zakęś (2005), ovulation following the application of Ovopel was observed 17–19 h (at 14.5 °C) later than after hCG was applied. However, those authors used a double injection of both hCG and Ovopel with an interval of 24 h between them, and the dose of Ovopel was smaller (1.25 pellet kg−1) than the one used in this experiment. Moreover, the females they used were at an earlier maturity stage (according to the description, it was probably stage II in the classification proposed by Żarski et al. 2012). GnRH analogues have been found to have a very short half-life period in fish (up to 23 min in gilthead seabream, Sparus aurata L.) (Gothilf and Zohar 1991). However, administering of GnRH increases the level of luteinising hormone (LH) in blood plasma, which actively participates in the FOM process (Mylonas et al. 1997), for as long as several days [13 days in the striped bass, Morone saxatilis (Walbaum)] (Mylonas et al. 1998). Since the level of LH during FOM shows an increasing tendency (Mylonas and Zohar 2001), the effectiveness of administration of GnRH and hCG, as well as the latency time following their application, may be closely related to the stage of female maturity. Kucharczyk et al. (2007) even suggested that hormonal stimulation in females in stage VI (following germinal vesicle breakdown) (as described by Żarski et al. 2012) is no longer necessary to obtain high-quality eggs after several hours.
It is noteworthy that hormonal stimulation did not significantly affect the basic parameter (motility) of the sperm obtained from males. This has been confirmed by other authors, who have reported that very good-quality sperm can be obtained from males during the reproductive period without any stimulation (Lappalainen et al. 2003; Zakęś and Demska-Zakęś 2005). It has great commercial importance because it may affect economic effectiveness of production positively where hormonal preparations as well as labour costs may be limited (Hakuć-Błażowska et al. 2009, 2010).
The thermal regimes applied following hormonal stimulation did not have any effect on the ovulation rate or egg quality after using any of the hormonal preparations. However, a significantly lower percentage of hatched larvae was found following application of CPH at 13 °C. Earlier reports have already mentioned a much lower quality of eggs obtained after the application of CPH as compared to the hCG among others in pikeperch (Kucharczyk et al. 2007) and in goldfish (Targońska and Kucharczyk 2011). It may be suggested that biological half-life, and in consequence activeness of the CPH, is much shorter than the hCG and in this way promotes the FOM less actively when fish are at the maturity stage III or IV. However, the reason for such difference remains unclear, and it has to be more closely studied. It is also noteworthy that application of PG-600 resulted in lower egg quality at both thermal regimes. It could be caused by lower activity of PMSG (contained in the PG-600 preparation) as compared to hCG. It was previously reported that activeness of hormonal preparations used for induction of ovulation may have significant effect on egg quality (Mylonas and Zohar 2001; Zohar and Mylonas 2001; Podhorec and Kouril 2009; Targońska et al. 2010).
The data published so far have shown that egg (and, consequently, larvae) quality depends closely on the type of hormonal preparation used (Żarski et al. 2009; Targońska et al. 2010). The highest survival rate in pikeperch has been achieved in many studies following the application of hCG as compared to Ovopel and CPH (Kucharczyk et al. 2007; Zakęś and Demska-Zakęś 2009). However, Zakęś and Demska-Zakęś (2005) after Ovopel application recorded an extreme low embryo survival rate (3.2 %), which is contrary to the results of this experiment. Therefore, it may again be suggested that the preparation dose and the females’ maturity stage may have a combined effect on the final outcome of reproduction. To date, different doses and numbers of injections of hCG have been tested for the pikeperch (Zakęś and Demska-Zakęś 2009). Apart from fragmentary data (Zakęś and Demska-Zakęś 2005; Kucharczyk et al. 2007), no detailed analyses have been published into the effect of different doses or the number of injections of the other commercially available hormonal preparations. Since the ovulation period (between the first and the last ovulated female) is very long following the application of the best preparations tested (hCG) (Zakęś and Demska-Zakęś 2009, this study), it is necessary to continue work on the effectiveness and efficiency of hormonal stimulation in the pikeperch. It is important in order to achieve complete and precise endocrinological control, which would enable improvement in biotechnology of the species reproduction, including reproduction outside the reproductive season, which is still being studied (Rónyai 2007; Zakęś and Demska-Zakęś 2009).
The results of this experiment have shown that it is possible to achieve desired effects by using a wide range of hormonal preparations, including GnRH analogues, provided the maturity stage of females is taken into account. However, further studies are needed to examine the effectiveness of different hormonal preparations, depending on the maturity stage and temperature, especially where high variability in maturity stage for wild percids was reported, even within one population and among fish caught at the same moment of the reproductive season (Żarski et al. 2012, b; the present study).
References
Baras E, Jobling M (2002) Dynamics of intracohort cannibalism in cultured fish. Aquacult Res 33:461–479
Baras E, Kestemont P, Melard C (2003) Effect of stocking density on the dynamics of cannibalism in sibling larvae of Perca fluviatilis under controlled conditions. Aquaculture 219:241–255
Bobe J, Labbe C (2010) Egg and sperm quality in fish. Gen Comp Endocrinol 165:535–548
Brzuska E (2003) Artificial spawning of female Polish line 3 carp (Cyprinus carpio L.) after treatment with pituitary homogenate and/or Ovopel. Aquacult Res 34:1321–1327
Cejko BI, Kowalski RK, Kucharczyk D, Targońska K, Krejszeff S, Żarski D, Glogowski J (2010) Influence of the length of time after hormonal stimulation on selected parameters of milt of ide Leuciscus idus L. Aquacult Res 41:804–813
Gothilf Y, Zohar Y (1991) Clearance of different forms of GnRH from the circulation of the gilthead seabream, Sparus aurata, in relation to their degradation and bioactivities. In: Scott AP, Sumpter JP, Kime DE, Rolfe MS (eds) Reproductive physiology of fish. Fish Symposium 91, Sheffield, pp 35–37
Hakuć-Błażowska A, Kupren K, Turkowski K, Targońska K, Jamróz M, Krejszeff S, Kwiatkowski M, Żarski D, Kucharczyk D (2009) Comparison of economic effectiveness of applying different hormonal agents in asp Aspius aspius (L.) and ide Leuciscus idus (L.). Pol J Nat Sci 24:224–234
Hakuć-Błażowska A, Kupren K, Turkowski K, Targońska K, Żarski D, Kucharczyk D (2010) A comparison of the economic effectiveness of various spawning agents for stimulating the reproduction of the cultured and wild forms of the common barbel Barbus barbus (L.). Pol J Nat Sci 25:272–286
Horvath L, Szabo T, Burke J (1997) Hatchery testing of GnRH analogue-containing pellets on ovulation in four cyprinid species. Pol Arch Hydrobiol 44:221–226
Kestemont P, Melard C (2000) Chapter 11. Aquaculture. In: Craig JF (ed) Percid fish—systematics, ecology and exploitation. Blackwell Science, Oxford, pp 191–224
Kestemont P, Xueliang X, Hamza N, Maboudou J, Imorou Toko I (2007) Effect of weaning age and diet on pikeperch larviculture. Aquaculture 264:197–204
Krejszeff S, Targońska K, Żarski D, Kucharczyk D (2009) Domestication affects spawning of the ide (Leuciscus idus)—preliminary study. Aquaculture 295:145–147
Krejszeff S, Targońska K, Żarski D, Kucharczyk D (2010a) Artificial reproduction of two different spawn-forms of the chub. Reprod Biol 10:67–74
Krejszeff S, Żarski D, Kucharczyk D, Kupren K, Targońska K, Mamcarz A (2010b) An experimental device for eggs incubation and fish larvae rearing under laboratory conditions. Pol J Nat Sc 25:190–199
Kucharczyk D, Kujawa R, Mamcarz A, Skrzypczak A, Wyszomirska E (1996) Induced spawning in perch, Perca fluviatilis L. using carp pituitary extract and HCG. Aquacult Res 27:847–852
Kucharczyk D, Kujawa R, Mamcarz A, Skrzypczak A, Wyszomirska E (1998) Induced spawning in perch, Perca fluviatilis L., using FSH + LH with pimozide or metoclopramide. Aquacult Res 29:131–136
Kucharczyk D, Kestemont P, Mamcarz A (2007) Artificial reproduction of pikeperch. Mercurius, Olsztyn 80 p
Kujawa R, Kucharczyk D, Mamcarz A (1999) A model system for keeping spawners of wild and domestic fish before artificial spawning. Aquacult Eng 20:85–89
Lappalainen J, Dörner H, Wysujack K (2003) Reproduction biology of pikeperch (Sander lucioperca (L.))—a review. Ecol Freshw Fish 12:95–106
Muller-Bellecke A, Zienert S (2008) Out-of-season spawning of pike perch (Sander lucioperca L.) without the need for hormonal treatments. Aquacult Res 39:1279–1285
Mylonas CC, Zohar Y (2001) Endocrine regulation and artificial induction of oocyte maturation and spermiation in basses of the genus Morone. Aquaculture 202:205–220
Mylonas CC, Scott AP, Zohar Y (1997) Plasma gonadotropin II, sex steroids, and thyroid hormones in wild striped bass (Morone saxatilis) during spermiation and final oocyte maturation. Gen Comp Endocrinol 108:223–236
Mylonas CC, Woods LC, Thomas P, Zohar Y (1998) Endocrine profiles of female striped bass (Morone saxatilis) during post-vitellogenesis, and induction of final oocyte maturation via controlled-release GnRHa-delivery systems. Gen Comp Endocrinol 110:276–289
Philipsen A (2008) Excellence fish: production of pikeperch in recirculating system. In: Fontaine P, Kestemont P, Teletchea F, Wang N (eds) Percid fish culture, from research to production. Presses Universitaires de Namur, Namur, p 67
Podhorec P, Kouril J (2009) Induction of final oocyte maturation in Cyprinidae fish by hypothalamic factors: a review. Vet Med 54:97–110
Rónyai A (2007) Induced out-of-season and seasonal tank spawning and stripping of pike perch (Sander lucioperca L.). Aquacult Res 38:1144–1151
Ronyai A, Lengyel SA (2010) Effects of hormonal treatments on induced tank spawning of Eurasian perch (Perca fluviatilis L.). Aquacult Res 41:e345–e347
Szczerbowski A, Kucharczyk D, Mamcarz A, Łuczyński MJ, Targońska K, Kujawa R (2009) Artificial off-season spawning of Eurasian perch Perca fluviatilis L. Arch Pol Fish 17:95–98
Targońska K, Kucharczyk D (2011) The application of hCG, CPH and Ovopel in successful artificial reproduction of goldfish (Carassius auratus auratus) under controlled conditions. Reprod Domest Anim (in press). doi:10.1111/j.1439-0531.2010.01723.x
Targońska K, Kucharczyk D, Kujawa R, Mamcarz A, Żarski D (2010) Controlled reproduction of asp, Aspius aspius (L.) using luteinizing hormone releasing hormone (LHRH) analogues with dopamine inhibitors. Aquaculture 306:407–410
Wang N, Xu X, Kestemont P (2009a) Effect of temperature and feeding frequency on growth performances, feed efficiency and body composition of pikeperch juveniles (Sander lucioperca). Aquaculture 289:70–73
Wang N, Mandiki SNM, Henrotte E, Bouyahia A-G, Mairesse G, Rougeot C, Mélard C, Kestemont P (2009b) Effect of partial or total replacement of forage fish by a dry diet on the quality of reproduction in pikeperch, Sander lucioperca. Aquacult Res 40:376–383
Yaron Z, Bogomolnaya A, Drori S, Biton I, Aizen J, Kulikovsky Z, Levavi-Sivan B (2009) Spawning induction in the carp: past experience and future prospects: a Review. Isr J Aquacult—Bamidgeh 61:5–16
Zakęś Z, Demska-Zakęś K (2005) Artificial spawning of pikeperch (Sander lucioperca (L.)) stimulated with human chorionic gonadotropin (hCG) and mammalian GnRH analogue with a dopamine inhibitor. Arch Pol Fish 13:63–75
Zakęś Z, Demska-Zakęś K (2009) Controlled reproduction of pikeperch Sander lucioperca (L.): a review. Arch Pol Fish 17:153–170
Żarski D, Kucharczyk D, Targońska K, Jamróz M, Krejszeff S, Mamcarz A (2009) Application of Ovopel and Ovaprim and their combinations in controlled reproduction of two reophilic cyprinid fish species. Pol J Nat Sc 24:235–244
Żarski D, Kucharczyk D, Sasinowski W, Targońska K, Mamcarz A (2010) The influence of temperature on successful reproductions of Burbot, Lota lota (L.) under hatchery conditions. Pol J Nat Sc 25:93–105
Żarski D, Bokor Z, Kotrik L, Urbanyi B, Horvath A, Targońska K, Krejszeff S, Palińska K, Kucharczyk D (2011a) A new classification of a pre-ovulatory oocyte maturation stage suitable for the synchronization of ovulation in controlled reproduction of Eurasian perch, Perca fluviatilis L. Rep Biol 11:194–209
Żarski D, Palińska K, Targońska K, Bokor Z, Kotrik L, Krejszeff S, Kupren K, Horvath A, Urbanyi B, Kucharczyk D (2011b) Oocyte quality indicators in Eurasian perch, Perca fluviatilis L., during reproduction under controlled conditions. Aquaculture 313:84–91
Żarski D, Kucharczyk D, Targońska K, Palińska K, Kupren K, Fontaine P, Kestemont P (2012) A new classification of pre-ovulatory oocyte maturation stages in pikeperch, Sander lucioperca (L.), and its application during artificial reproduction. Aquacult Res 43:713–721
Zohar Y, Mylonas CC (2001) Endocrine manipulations of spawning in cultured fish: from hormones to genes. Aquaculture 197:99–136
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Żarski, D., Targońska, K., Kaszubowski, R. et al. Effect of different commercial spawning agents and thermal regime on the effectiveness of pikeperch, Sander lucioperca (L.), reproduction under controlled conditions. Aquacult Int 21, 819–828 (2013). https://doi.org/10.1007/s10499-012-9597-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-012-9597-2