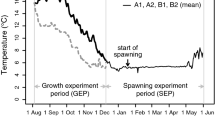
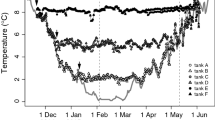
Abstract
Culture of Ruditapes decussatus is clearly limited by the availability of seed, as this production proceeds almost exclusively from natural recruitment. Artificial spawning and larval rearing programs could provide an alternative source of spat. This study was designed to evaluate the effect of different conditioning temperatures on the broodstock maturation, spawning success and larval viability of two geographically (north and south of the Iberian Peninsula) distinct populations of European clam (R. decussatus) collected at different periods of the year in order to create “optimal” artificial spawning and larval rearing programs. Two batches of clams from each population were collected in October and February, and conditioned at 18 ± 1°C, 20 ± 1°C and 22 ± 1°C. Of the three variables analysed the timing of broodstock collection was the most determining factor for gametogenic development, spawning and larval rearing. Geographic origin and conditioning temperature also greatly affected the spawning. The results also showed that the February conditioning was more effective than October and that the best conditioning temperatures were 20 ± 1°C and 22 ± 1°C for the northern and southern populations, respectively. These results suggest that the efficient conditioning temperature for each population of the same species is related to the seasonal temperature regime from their geographic origin. Larval viability and growth performance seemed to be independent of the broodstock conditioning.



Similar content being viewed by others
Abbreviations
- AFDW:
-
Ash free dry weight
- ANOVA:
-
Analysis of variance
References
Andersen S, Ringvold H (2000) Seasonal differences in effect of broodstock diet on spawning success in the great scallop. Aquacult Int 8:259–265. doi:10.1023/A:1009279422686
Ansell AD, Frenkiel L, Moueza M (1980) Seasonal changes in tissue weight and biochemical composition for the bivalve Donax trunculus L on the Algerian coast. J Exp Mar Biol Ecol 45:105–116. doi:10.1016/0022-0981(80)90073-8
Avendaño M, Le Pennec M (1997) Intraspecific variation in gametogenesis in two populations of the Chilean molluscan bivalve, Argopecten purpuratus (Lamarck). Aquacult Res 28:175–182. doi:10.1111/j.1365-2109.1997.tb01030.x
Barber BJ, Blake NJ (1985) Intra-organ biochemical transformations associated with oogenesis in the bay scallop, Argopecten irradians concentricus (Say), as indicated by 14C incorporation. Biol Bull 168:39–49. doi:10.2307/1541172
Delgado M, Pérez-Camacho A (2002) Efectos de la rácion de alimento en el desarrollo gonadal de la almeja Ruditapes decussatus (L.). Bol Inst Esp Oceanogr 18(1–4):293–300
Delgado M, Pérez-Camacho A (2005) Histological study of the gonadal development of Ruditapes decussatus (L.) (Mollusca Bivalvia) and its relationship with available food. Sci Mar 69(1):87–97
Delgado M, Pérez-Camacho A (2007) Comparative study of gonadal development of Ruditapes philippinarum (Adam and Reeve) and Ruditapes decussatus (L.) (Mollusca: Bivalvia): Influence of temperature. Sci Mar 71(3):471–484
Delgado M, Pérez-Camacho A, Labarta U, Fernández-Reiriz MJ (2004) The role of lipids in the gonadal development of the clam Ruditapes decussatus (L.). Aquaculture 241(1–4):395–411. doi:10.1016/j.aquaculture.2004.07.018
DGPA (2006) Recursos da Pesca. Série Estatística 2005. DGPA, Lisboa, 19 A-B, p 163
Devauchelle N, Mingant C (1991) Review of the reproductive physiology of the scallop, Pecten maximus, applicable to intensive aquaculture. Aquat Living Resour 4:41–51. doi:10.1051/alr:1991004
Falcão M (1997) Dinâmica dos nutrientes na Ria Formosa: efeitos da interacção da laguna com as suas interfaces na reciclagem do azoto, fósforo e sílica. Dissertation, University of Algarve, Portugal, p 218
Folch J, Less M, Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Gabbot PA (1975) Storage cycles in marine bivalves molluscs: a hypothesis concerning the relationship between glycogen and gametogenesis. In: Barnes H (ed) Proceedings of the Ninth Eur Mar Biol Symp. Aberdeen University Press, Abeerden, pp 191–211
Gallager SM, Mann R (1986) Growth and survival of larvae of Mercenaria mercenaria (L.) and Crassostrea virginica (Gmelin) relative to broodstock conditioning and lipid content of eggs. Aquaculture 56:105–112. doi:10.1016/0044-8486(86)90021-9
Hamida L, Medhiouband MN, Cochard JC, Rhomdhane MS, Le Pennec M (2004) Étude comparative du cycle de reproduction de la palourde Ruditapes decussatus en milieu naturel (sud Tunisie) et contrôlé (écloserie). Cah Biol Mar 45:291–303
Iglesias JIP, Camacho C, Navarro E, Labarta U, Beiras R, Hawkins AJS et al (1996) Microgeographic variability in feeding, absorption and condition of mussels (Mytilus galloprovincialis Lmk): a transplant experiment. J Shellfish Res 15(3):673–680
Kennedy VS, Newel RIE, Eble AF (1996) The eastern oyster Crassostrea virginica. A Maryland Sea Grant Book, College Park, p 734
Lannan JE, Robinson A, Breese WP (1980) Broodstock management of Crassostrea gigas II. Broodstock conditioning to maximise larval survival. Aquaculture 21:337–345. doi:10.1016/0044-8486(80)90068-X
Laruelle F, Guillou J, Paulet YM (1994) Reproductive pattern of clams, Ruditapes decussatus and R. phillippinarum on intertidal flats in Brittany. J Mar Biol Assoc UK 74(2):351–366
Le Pennec M, Robert R, Avendaño M (1998) The importance of gonadal development on larval production in pectinids. J Shellfish Res 17:97–101
Lucas A, Benninger P (1985) The use of physiology condition indices in marine bivalves aquaculture. Aquaculture 44:187–200. doi:10.1016/0044-8486(85)90243-1
Marsh JB, Weinstein DB (1966) Simple charring method for determination of lipids. J Lipid Res 7:574–576
Martínez G, Aguilera C, Mettifogo L (2000) Interactive effects of diet and temperature on reproductive conditioning of Argopecten pupuratus broodstock. Aquaculture 183:149–159. doi:10.1016/S0044-8486(99)00291-4
Martoja R, Martoja M (1967) Initiations aux techniques de l’histologie animale. Masson et Cie (ed), Paris, p 345
Mladineo I, Peharda M, Orhanović S, Bolotin J, Pavela-Vrančić M, Treursić B (2007) The reproductive cycle, condition index and biochemical composition of the horse-bearded mussel Modiolus barbatus. Helgol Mar Res 61:183–192. doi:10.1007/s10152-007-0065-8
Muranaka MS, Lannan JE (1984) Broodstock management of Crassostrea gigas: environmental influences on broodstock conditioning. Aquaculture 39:217–228. doi:10.1016/0044-8486(84)90267-9
Ojea J, Pazos AJ, Martínez D, Novoa S, Sánchez JL, Abad M (2004) Seasonal variation in weight and biochemical composition of the tissues of Ruditapes decussatus in relation to the gametogenic cycle. Aquaculture 238:451–468. doi:10.1016/j.aquaculture.2004.05.022
Pacheco L, Vieira A, Ravasco J (1989) Crescimento e reprodução de Ruditapes decussatus na Ria Formosa (Sul de Portugal). Bentos 6:129–136
Pérez-Camacho A (1980) Biologia de Venerupis pullastra (Montagu, 1803) e Venerupis decussata (Linné, 1767) (Mollusca, Bivalvia) con especial referencia a los factores determinantes de la producción. Bol Inst Esp Oceanogr 281:353–358
Pérez-Camacho A, Delgado M, Fernández-Reiriz MJ, Labarta U (2003) Energy balance, gonad development and biochemical composition in the clam Ruditapes decussatus. Mar Ecol Prog Ser 258:133–145. doi:10.3354/meps258133
Rodriguez-Moscoso E, Arnaiz R (1998) Gametogenesis and energy storage in a population of grooved carpet-shell clam, Tapes decussatus (Linne, 1787), in northwest Spain. Aquaculture 162(1–2):125–139. doi:10.1016/S0044-8486(98)00170-7
SAS Institute Inc. (2000) SAS OnlineDOC®. Version eight (CD ROM)
Sastry AN (1975) Physiology and ecology of reproduction in marine invertebrates. In: Vernberg FJ (ed) Physiological ecology of estuarine organisms. University of South Carolina Press, Columbia, pp 279–299
Shafee MS, Daoudi M (1991) Gametogenesis and spawning in the carpet-shell clam, Ruditapes decussatus (L.) (Mollusca: Bivalvia), from the Atlantic coast of Morocco. Aquacult Fish Manage 22:203–216
Silva A, Novoa S, Sordo A, Abad M, Sánchez JL (2002) The influence of photoperiod on hatchery conditioning of broodstock of the clam Ruditapes decussatus. Europ Aquacult Soc Spec Publ 32:479–480
STAT SOFT INC. (1993) Statistic for Windows, release 4.5, Copy right
Trigui-El-Menif N, Le Pennec M, Maamouri F (1995) Reproduction of the European Clam Ruditapes decussatus (mollusc, bivalve) along the Tunian coasts. Mar Life 5(1):35–42
Utting SD, Millican PF (1997) Techniques for the hatchery conditioning of bivalve broodstocks and the subsequent effect on egg quality and larval viability. Aquaculture 155:45–54. doi:10.1016/S0044-8486(97)00108-7
Vilela H (1950) Vida bentónica de Tapes decussatus. Trav Sta Biol Mar Lisb 53:1–79
Viles FJ, Silverman L (1949) Determination of starch and cellulose with anthrone. J Anal Chem 21:950–953. doi:10.1021/ac60032a019
Walne PR (1974) Culture of bivalves molluscs, 50 years of experience at Conway. Fishing News Books, West Byfleet, p 173
Walne PR, Mann R (1975) Growth and biochemical composition of Ostrea edulis and Crassostrea gigas. In Barnes H (ed) Proceedings of the 9th Eur Mar Biol Symp Oban (Scotlland), pp 587–607
Acknowledgements
The authors are grateful to Dr. M. Castro for reviewing and improving the statistical treatments and to J. White for revising the English. Gratitude is also extended to António Machado, who was in charge of the broodstock.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matias, D., Joaquim, S., Leitão, A. et al. Effect of geographic origin, temperature and timing of broodstock collection on conditioning, spawning success and larval viability of Ruditapes decussatus (Linné, 1758). Aquacult Int 17, 257–271 (2009). https://doi.org/10.1007/s10499-008-9197-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-008-9197-3