Abstract
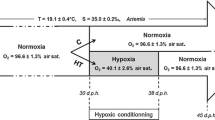
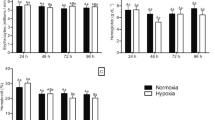
Compensatory growth following stress is a strategy aquatic animals use to adjust themselves to a variable environment. Studies on the recovery growth of aquatic animals are not only of theoretical value in ecophysiology and evolution, but also important to applications in aquaculture and fisheries resource management. In this experiment, juvenile Fenneropenaeus chinensis with an initial average body weight of about 3.72 g were exposed to hypoxic water (about 2.08 and 3.11 mg/l of dissolved oxygen (DO) content) for 10 days and then switched to normoxic water (about 5.63 and 5.59 mg/l DO). Compared with juveniles in normoxia, juveniles in the hypoxia period allocated a greater proportion of energy to metabolism and exuviations but allocated less energy to daily metabolism per gram shrimp weight (J/g/day). This reduced feed conversion efficiency and feeding rate. Finally, F. chinensis suffered growth depression. The juveniles completely compensated for hypoxia-induced growth depression in 30 days after being switched into normoxic water and the compensation was achieved mainly by hyperphagia and slightly by improvement of feed conversion efficiency. During the recovery period the hypoxic-stressed shrimp showed higher daily metabolic energy (J/g/day) than controls (P < 0.05). Which means the stressed shrimp had more energy for feeding-related activities. So hyperphagia was observed. Energy analysis indicated that F. chinensis improved feed-conversion efficiency mainly by reducing the percentage of energy lost in feces and exuviations. The results showed that short-term non-lethal hypoxia would not affect the growth of juvenile F. chinensis if there were enough time for the stressed shrimp to recover. This suggested F. chinensis was capable of adapting to DO fluctuation to some extent and short-term non-lethal hypoxia would not have an obvious effect on natural, released, and cultured shrimp stock.

Similar content being viewed by others
References
Bayer R, Riley J, Donahue D (1998) The effect of dissolved oxygen levels on the weight gain and shell hardness of new-shell American lobster, Homarus americanus. J World Aqua Soc 29(4):491–493
Bilton HT, Robins GL (1973) The effect of starvation and subsequent feeding on survival and growth of Fulton Channel sockeye salmon fry, Oncorhynchus nerka. J Fish Res Board Can 30(1):1–5
Blake RW, Chan KHS (2006) Cyclic feeding and subsequent compensatory growth do not significantly impact standard metabolic rate or critical swimming speed in rainbow trout. J Fish Biol 69(3):818–827
Bostworth BG, Wolters WR (1995) Compensatory growth in juvenile red swamp crayfish, Procambarus clarkii. Freshw Crayfish 1:648–656
Boyle PC, Storlien LH, Harper AE, Keesey RE (1981) Oxygen consumption and locomotor activity during restricted feeding and realimentation. Am J Physiol 241:392–397
Brooke OG, Ashworth A (1972) The influence of malnutrition on the postprandial metabolic rate and respiratory quotient. Br J Nutr 27:407
Bull CD, Metcalf NB (1997) Regulation of hyperphagia in response to varying energy deficits in overwintering juvenile Atlantic salmon. J Fish Biol 50:498–510
Chen N, Yang X, Li X, Liu H, Lin G, Li F, Zhang H, Lin L (translators) (1992) The biology of the peaeidae (Original book edited by Dall W, Hill BJ, Rothlisberg PC, Starples DJ 1990). Ocean University of Qingdao Press, Qingdao, 232 pp
Clark JV (1986) Inhibition of moulting in Penaeus semisulcatus (De Haan) by long-term hypoxia. Aquaculture 52(4):253–254
Coiro LL, Poucher SL, Miller DC (2000) Hypoxic effects on growth of Palaemonetes vulgaris larvae and other species: using constant exposure data to estimate cyclic exposure response. J Exp Mar Biol Ecol 247(2):243–255
Cui Y (1989) Bioenergetics of fishes: theory and methods. Acta Hydrobiol Sin (in Chinese) 13:369–383
Dobson SH, Holmes RM (1984) Compensatory growth in the rainbow trout, Salmo gairdneri Richardson. J Fish Biol 25:649–656
Elliott JM (1976) Energy lost in the waste products of brown trout (Salmo trutta L.). J Anim Ecol 45(2):561–580
Fotheringham N, Weissberg GH (1979) Some causes, consequences and potential environmental impacts of oxygen depletion in the Northern Gulf of Mexico. In: Proceedings of eleventh annual offshore technology conference, Houston, TX, April 30–May 3, 1979, vol 4, pp 2205–2208
Freeman BM (1964) The effect of diet and breed upon the oxygen requirements of the domestic fowl during the first fortnight of post-embryonic life. Br Poul Sci 5:263
Ganga U, James PSBR (1993) Influence of different levels of ambient oxygen on growth and metabolite changes in laboratory reared Penaeus indicus. CMFRI special publication, Cochin, India
Guppy M, Fuery CJ, Flanigan JE (1994) Biochemical principles of metabolic depression. Comp Biochem Physiol 109B:175–189
Hayward RS, Noltie DB, Wang N (1997) Use of compensatory growth to double hybrid sunfish growth rates. Trans Am Fish Soc 126:316–322
Heide A, Foss A, Stefansson SO, Mayer I, Norberg B, Roth B, Jenssen MD, Nortvedt R, Imsland AK (2006) Compensatory growth and fillet crude composition in juvenile Atlantic halibut: effects of short term starvation periods and subsequent feeding. Aquaculture 261(1):109–117
Hogendoorn H (1983) Growth and production of African catfish, Clarias lazera (C. and V.). III. Bioenergetic relations of body weight and feeding level. Aquaculture 35:1–7
Jobling M (1994) Fish bioenergetics. Chapman & Hall, London, pp 107–109
Jobling M, Meløy OH, Santos J, Christiansen B (1994) The compensatory growth response of the Atlantic cod: effects of nutritional history. Aquacult Int 2(2):75–90
Kang JC, Matsuda O (1994) Combined effects of hypoxia and hydrogen sulfide on early developmental stages of white shrimp Metapenaeus monoceros. Appl Biol Sci 33(1):21–27
Kang JC, Matsuda O, Imamura N (1995) Effects of hypoxia and hydrogen sulfide on survival of the prawn Macrobrachium nipponense in Lake Kojima, Japan. Nipp Suis Gakk 61(6):821–826
Kennish MJ (2002) Environmental threats and environmental future of estuaries. Environ Conserv 29(1):78–107
Kim MK, Lovell RT (1995) Effects of restricted feeding regimens on compensatory weight gain and body tissue changes in channel catfish Ictalurus punctatus in ponds. Aquaculture 135:285–293
Kiørboe T, Munk P, Richardson K (1987) Respiration and growth of larval herring Clupea harengus: relation between specific dynamic action and growth efficiency. Mar Ecol Prog Ser 40:1–10
Klein Breteler WCM (1975) Food consumption, growth and energy metabolism of juvenile shore crab (Carcinus maenas). Neth J Sea Res 9:255–272
Le-Francois NR, Blier PU, Adambounou LT, Lacroix M (1999) Exposures to low-level ionizing radiation: effects of biochemical and whole-body indices of growth in juvenile brook charr (Salvelinus fantinalis). J Exp Zool 283(3):315–325
Lemos D, Phan VN (2001) Energy partitioning into growth, respiration, excretion and exuviae during larval development of the shrimp Farfantepenaeus paulensis. Aquaculture 199:131–143
Levine DM, Sulkin SD (1979) Partitioning and utilization of energy during the larval development of the xanthid crab, Rithropanopeus harrisii (Gould). J Exp Mar Biol Ecol 40:247–257
Lin X, Zhou X, Yu H, Lin J, Xu Z (2004) The effects of starvation on biochemical composition and compensatory growth in Penaeus vannamei. J Fish China 28(1):47–53
McGraw W, Teichert-Coddington DR, Rouse DB, Boyd CE (2001) Higher minimum dissolved oxygen concentrations increase penaeid shrimp yields in earthen ponds. Aquaculture 199(3–4):311–321
Miglvas I, Jobling M (1989) The effects of feeding regime on proximate body composition and patters of energy deposition in juvenile Arctic charr, Salvelinus alpinus. J Fish Biol 35:1–11
Montserrat N, Gabillard JC, Capilla E, Navarro MI, Gutiérrez J (2007) Role of insulin, insulin-like growth factors, and muscle regulatory factors in the compensatory growth of the trout (Oncorhynchus mykiss). Gen Comp Endocrinol 150(3):462–472
Mortensen A, Damsgård B (1993) Compensatory growth and weight segregation following light and temperature manipulation of juvenile Atlantic salmon (Salmo salar L.) and Arctic charr (Salvelinus alpinus L.). Aquaculture 114:261–272
Nicieza A, Metcalfe NB (1997) Growth compensation in juvenile Atlantic salmon: responses to depressed temperature and food availability. Ecology 78:2385–2400
Ocampo Victoria L (1998) Effect of dissolved oxygen and temperature on the growth, respiratory metabolism and energetics of brown shrimp (Penaeus californiensis) juveniles. Cent. de Investigaciones Biologicas del Noroeste, La Paz, Baja California Sur, (Mexico), 32 pp., Sep. 1998
Pavela JS, Ross JL, Chittenden ME Jr (1983) Sharp reductions in abundance of fishes and benthic macroinvertebrates in the Gulf of Mexico off Texas associated with hypoxia. Northeast Gulf Sci 6(2):167–173
Quinton JC, Blake RW (1990) The effects of feed cycling and ration level on the compensatory growth response in rainbow trout, Oncorhynchus mykiss. J Fish Biol 37:33–41
Renaud ML (1986) Detecting and avoiding oxygen deficient sea water by brown shrimp, Penaeus aztecus (Ives), and white shrimp Penaeus setiferus (Linnaeus). J Exp Mar Biol Ecol 98(3):283–292
Rosas C, Sanchez A, Díaz-Iglesia E, Brito R, Martinez E, Soto LA (1997) Critical dissolved oxygen level to Penaeus setiferus and P. schmitti postlarvae (PL10–18) exposed to salinity changes. Aquaculture 152:259–272
Rosas C, Martinez E, Gaxiola G, Brito R, Díaz-Iglesia E, Soto LA (1998) Effect of dissolved oxygen on the energy balance and survival of Penaeus setiferus juveniles. Mar Ecol Prog Ser 174:67–75
Rosas C, Martinez E, Gaxiola G, Brito R, Díaz-Iglesia E, Soto LA (1999) The effect of dissolved oxygen and salinity on oxygen consumption, ammonia excretion and osmotic pressure of Penaeus setiferus (Linnaeus) juveniles. J Exp Mar Biol Ecol 234:41–57
Russell NR, Wootton RJ (1992) Appetite and growth compensation in European minnows (Phoxinus phoxinus) following short periods of food restriction. Environ Biol Fish 34:277–285
Russell NR, Wootton RJ (1993) Satiation, digestive tract evacuation and return of appetite in the Europe minnow, Phoxinus phoxinus (Cyprinidae) following short period of pre-prandial starvation. Environ Biol Fish 38:385–390
Tacon AGJ, Rodrigues AMP (1984) Comparison of chromic oxide, crude fibre, polyethylene and acid-insoluble ash as dietary markers for the estimation of apparent digestibility coefficients in rainbow trout. Aquaculture 43(4):391–399
Thetmeyer H, Waller U, Black KD, Inselmann S, Rosenthal H (1999) Growth of European sea bass (Dicentrachus labrax L.) under hypoxic and oscillating oxygen conditions. Aquaculture 174:355–367
Wang Y, Zhang H, Qi Z (1998) Occurrence and effects of harmful bloom caused by Prorocentrtum micans in seawater experimental enclosures. J Fish China 22(3):218–222
Wang J, Li Z, Xie S (2005) Effects of starvation on growth and biochemical composition in shrimp Macrobrachium niponense. J Hebei Univer (Natural Science Edition) 25(6):644–649
Weatherley AH, Gill HS (1981) Recovery growth following periods of restricted rations and starvation in rainbow trout, Salmo gairdneri Richardson. J Fish Biol 18:195–208
Wilson PN, Osbourn DF (1960) Compensatory growth after undernutrition in mammals and birds. Biol Rev 35:324–363
Wootton RJ (1990) Ecology of teleost fishes. Chapman and Hall, London
Wu L, Dong S (2001) The effects of repetitive ‘starvation-and-refeeding’ cycles on the compensatory growth response in Chinese shrimp, Fenneropenaeus chinensis (Osbeck, 1765) (Decapoda, Penaeidae). Crustaceana 74(11):1225–1239
Wu L, Dong S (2002) Compensatory growth responses in juvenile Chinese shrimp, Fenneropenaeus chinensis, at different temperatures. J Crustacean Boil 22(3):511–520
Wu L, Dong S, Wang F, Tian X (2000) Compensatory growth response following periods of starvation in Chinese shrimp, Penaeus chinensis Osbeck. J Shellfish Res 19(2):717–722
Wu L, Dong S, Tian X (2001a) The compensatory growth in the Chinese shrimp (Penaeus chinensis) following starvation. Acta Ecol Sin 21(3):452–457
Wu L, Dong S, Wang F, Tian X, Ma S (2001b) The effect of previous feeding regimes on the compensatory growth response in Chinese shrimp, Fenneropenaeus chinensis. J Crustacean Boil 21(3):559–565
Xie X, Sun R (1992) The daily total metabolism and specific dynamic action in the southern catfish (Silurus meridionalis). Acta Hydrobiol Sin 16(3):200–207
Yambayamba ESK, Price MA, Foxcroft GR (1996) Hormonal status, metabolic changes and resting metabolic rate in beef heifers undergoing compensatory growth. J Am Sci 74:57–69
Yamochi S, Sano M (1992) Changes of dissolved oxygen concentration and its effect on the mortality of southern rough shrimp at Tanigawa fishing port, Osaka Bay. Bull Jpn Soc Fish Oceanogr 56(1):1–12
Zaitsev YP (1992) Recent changes in the tropic structure of the Black Sea. Fish Oceanogr 1(2):180–189
Zhu X, Miao F, Xian W (2001) The effects of compensatory growth on patterns of fisheries ecology. J Fish China 25(3):265–269
Acknowledgement
This work was supported by the grants from the National Key Technologies R & D Programme of China during the 10th Five-Year Plan Period (No. 2004BA526B0201), the National Key Technology R & D Program of China during the 11th Five-Year Plan Period (No. 2006BAD09A15), and the Key Laboratory of Mariculture of the Ministry of Education, Ocean University of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wei, LZ., Zhang, XM., Li, J. et al. Compensatory growth of Chinese shrimp, Fenneropenaeus chinensis following hypoxic exposure. Aquacult Int 16, 455–470 (2008). https://doi.org/10.1007/s10499-007-9158-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-007-9158-2