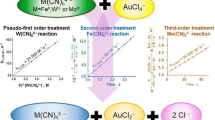
Abstract
Reactions between cyanide and compounds, which contain S–S bonds, in aqueous media result in formation of thiocyanate. In this work, we studied the kinetics of reactions of thiosulfate with free cyanide and its complexes under environmental conditions. Rates of reactions between cyanide species and thiosulfate decrease in the following order: CN− > HCN > [Fe(CN)6]3− > [Fe(CN)6]4−. However, at neutral and slightly acidic pH range, reaction of thiosulfate with iron-cyanide complexes outcompetes its reaction with free cyanide, which exists in equilibrium with complexed cyanide. At environmentally relevant conditions, the characteristic time of reaction between free cyanide and thiosulfate was found to be tens of thousands of years, while for iron-cyanide complexes it was found to be hundreds to millions of years. Examples of application of kinetic parameters for calculation of rates of cyanide consumption in industrial (coke oven wastewater) and non-polluted natural aquatic system (Delaware Great Marsh) are provided.





Similar content being viewed by others
References
Bak F, Schuhmann A, Jansen K (1993) Determination of tetrathionate and thiosulfate in natural samples and microbial cultures by a new, fast and sensitive ion chromatographic technique. FEMS Microbiol Ecol 12:257–264. https://doi.org/10.1111/j.1574-6941.1993.tb00038.x
Bartlett PD, Davis REJ (1958) Reactions of elemental sulfur. II. The reaction of alkali cyanides with sulfur, and some single-sulfur transfer reactions. Am Chem Soc 80:2513
Blonder B, Boyko V, Turchyn AV, Antler G, Sinichkin U, Knossow N, Klein R, Kamyshny A Jr (2017) Impact of aeolian dry deposition of reactive iron minerals on sulfur cycling in sediments of the Gulf of Aqaba. Front Microbiol 8:1131
Chen KY, Morris JC (1972) Kinetics of oxidation of aqueous sulfide by O2. Environ Sci Technol 6(6):529–537
Dash RR, Gaur A, Balomajumder C (2009) Cyanide in industrial wastewaters and its removal: a review on biotreatment. J Hazard Mater 163:1–11
Davis RE (1962) Displacement reactions at the sulfur atom. III. The reaction of cyanide with thiosulfate. J Phys Chem 66:956
Donato DB, Nichols O, Possingham H, Moore M, Ricci PF, Noller B (2007) A critical review of the effects of gold cyanide-bearing tailings solutions on wildlife. Environ Int 33(7):974–984
Dowler MJ, Ingmanson DE (1979) Thiocyanate in Red Sea brine and its implications. Nature 279:51–52
Dzombak DA, Ghosh RS, Young TC (2006) In: Dzombak DA, Ghosh RS, Wong-Chong GM (eds) Cyanide in Water and Soil: chemistry, risk, and management. CRC Press, Boca Raton, pp 57–92
Findlay AJ, Kamyshny A (2017) Turnover rates of intermediate sulfur species (S 2−x , S0, S2O3 2−, S4O6 2−, SO3 2−) in anoxic freshwater and sediments. Front Microbiol 8:2551
Gensemer RW, DeForest DK, Stenhouse AJ, Higgins CJ, Cardwell RD (2006) In: Dzombak DA, Ghosh RS, Wong-Chong GM (eds) Cyanide in water and soil: chemistry, risk, and management. CRC Press, Boca Raton, pp 251–284
Ghosh W, Dam B (2009) Biochemistry and molecular biology of lithotrophic sulfur-oxidation by taxonomically and ecologically diverse bacteria and archaea. FEMS Microbiol Rev 33:999–1043
Ghosh RS, Dzombak DA, Luthy RG, Nakles DV (1999) Subsurface fate and transport of cyanide species at a manufactured-gas plant site. Water Environ Res 71:1205
Gould WD, King M, Mohapatra BR, Cameron RA, Kapoor A, Koren DW (2012) A critical review on destruction of thiocyanate in mining effluents. Miner Eng 34:38–47
Jaszczak E, Polkowska Ż, Narkowicz S, Namieśnik J (2017) Cyanides in the environment—analysis—problems and challenges. Environ Sci Pollut Res 24:15929–15948. https://doi.org/10.1007/s11356-017-9081-7
Jørgensen BB, Fossing H, Wirsen CO, Jannasch HW (1991) Sulfide oxidation in the anoxic Black Sea chemocline. Deep Sea Res 38(Suppl 2):S1083–S1103
Kamyshny A (2009) Improved cyanolysis protocol for detection of zero-valent sulfur in natural aquatic systems. Limnol Oceanogr: Methods 7:442–448
Kamyshny A, Zerkle AL, Mansaray ZF, Ciglenečki I, Bura-Nakić E, Farquhar J, Ferdelman TG (2011) Biogeochemical sulfur cycling in the water column of a shallow stratified sea-water lake: speciation and quadruple sulfur isotope composition. Mar Chem 127:144–154
Kamyshny A, Oduro H, Mansaray ZF, Farquhar J (2013) Hydrogen cyanide accumulation and transformations in non-polluted salt marsh sediments. Aquat Geochem 19:97–113
Kamyshny A, Druschel G, Mansaray ZF, Farquhar J (2014) Multiple sulfur isotopes fractionation evidence of abiotic sulfur transformations in Yellowstone National Park geothermal springs. Geochem Trans 15:7
Karavaiko GI, Kondrat’eva EE, Savari EE, Grigor’eva NV, Avakyan ZA (2000) Microbial degradation of cyanide and thiocyanate. Microbiology 69(2):167–173
Karchmer JK (1970) Analytical chemistry of sulfur and its compounds. Wiley, Hoboken
Knossow N, Blonder B, Eckert W, Turchyn AV, Antler G, Kamyshny A Jr (2015) Annual sulfur cycle in a warm monomictic lake with sub-millimolar sulfate concentrations. Geochem Trans 16:7
Koh T (1990) Analytical chemistry of polythionates and thiosulfate. Anal Sci 6:3–14
Koppenol WH (1980) Effect of a molecular dipole on the ionic strength dependence of a bimolecular rate constant. Biophys J 29:493–508
Kurashova I, Halevy I, Kamyshny A Jr (2018) Kinetics of decomposition of thiocyanate in natural aquatic systems. Environ Sci Technol 52:1234–1243. https://doi.org/10.1021/acs.est.7b04723
Li Q, Jacob DJ, Bey I, Yantosca RM, Zhao Y, Kondo Y, Notholt J (2000) Atmospheric hydrogen cyanide (HCN): biomass burning source, ocean sink? Geophys Res Lett 27:357–360
Luther GW III, Ferdelman TG, Kostka JE, Tsamakis EJ, Church TM (1991) Temporal and spatial variability of reduced sulfur species (FeS2, S2O3 2−) and porewater parameters in salt marsh sediments. Biogeochem 14:57–88
Luthy RG, Bruce SG Jr (1979) Kinetics of reaction of cyanide and reduced sulfur species in aqueous solutions. Enviro. Sci Technol 13:1481–1487
Meeussen JCL, Keizer MG, De Haan FAM (1992) The chemical stability and decomposition rate of iron cyanide complexes in soil solutions. Environ Sci Technol 26(3):511–516
Mishra L, Paul KK, Jena S (2018) Characterization of coke oven wastewater. Earth Envirol Sci 167:012011
Oluwole ASA, Onabolu AO, Cotgreave IA, Rosling H, Persson A, Link H (2003) Influence of endemic ataxic polyneuropathy and its relation to exposure to cyanide in a Nigerian community. J Neurol Neurosurg Psychiatry 74:1417–1422
Oulego P, Laca A, Diaz M (2013) Kinetics and pathways of cyanide degradation at high temperatures and pressures. Environ Sci Technol 47:1542–1549
Oulego P, Collado S, Laca A, Diaz M (2014) Simultaneous oxidation of cyanide and thiocyanate at high pressure and temperature. J Hazard Mater 280:570–578
Pal P, Kumar R (2014) Treatment of coke wastewater: a critical review for developing sustainable management strategies. Sep Purif Rev 43:89–123
Rong L, Lim L, Takeuchi T (2005) Determination of iodide and thiocyanate in seawater by liquid chromatography with poly(ethylene glycol) stationary phase. Chromatographia 61:371–374
Schippers A (2004) Biogeochemistry of metal sulfide oxidation in mining environments, sediments, and soils. In: Amend JP, Edwards KJ, Lyons TW (eds) Sulfur biogeochemistry—past and present. Special paper, vol 379. Geological Society of America, Boulder, pp 49–62
Schippers A, Jørgensen BB (2002) Biogeochemistry of pyrite and iron sulfide oxidation in marine sediments. Geochim Cosmochim Acta 66:85–92
Sehmel GA (1989) Cyanide and antimony thermodynamic database for the aqueous species and solids for the EPA-MINTEQ geochemical code, PNL-6835. Pacific Northwest Laboratory, Richland
Sekerka I, Lechner JF (1976) Potentiometric determination of low levels of simple and total cyanides. Water Res 10:479–483
Smith A, Mudder T (2001) Chemistry and treatment of cyanidation wastes. Mining J. Books, London
Souren AWMG (2000) Living with cyanide. Geochem News 105:16–26
Steudel R (1996) Mechanisms of the formation of elemental sulfur from aqueous sulfide in chemical and microbiological desulfurization processes. Ind Eng Chem Res 35:1417–1423
Stumm W, Morgan JJ (1996) Aquatic chemistry, 3rd edn. Wiley, New York
Szekers L (1974) Analytical chemistry of sulfur acids. Talanta 21:1–44
Sӧrbo B (1957) A colorimetric method for the determination of thiosulfate. Biochim Biophys Acta 23:412
Takasaki Y, Satoh H, Onuki M, Mino T, Ito K, Miki O (2007) Behavior of nitrite oxidizers in the nitrification/denitrification process for the treatment of simulated coke-oven wastewater. J Water Environ Technol 5:29–36
Taube H (1943) Disappearance of thiosulfate in solutions of maleic acid; catalysis of cis-trans isomerization. Am Chem J 65:526–531
Upadhyay SK (2006) Chemical kinetics and reaction dynamics. Springer, New York
Urban PJZ (1961) Colorimetry of sulphur anions. I. An improved colorimetric method for the determination of thiosulphate. Anal Chem 179:415
Watts MP, Gan HM, Peng LY, Lê Cao K-A, Moreau JW (2017) In situ stimulation of thiocyanate biodegradation through phosphate amendment in gold mine tailings water. Environ Sci Technol 51:13353–13362
Wong-Chong GM, Dzombak DA, Gjosh RS (2006a) In: Dzombak DA, Ghosh RS, Wong-Chong GM (eds) Cyanide in water and soil: chemistry, risk, and management. CRC Press, Boca Raton, pp 1–14
Wong-Chong GM, Ghosh RS, Bushey JT, Ebbs SD, Neuhauser EF (2006b) In: Dzombak DA, Ghosh RS, Wong-Chong GM (eds) Cyanide in water and soil: chemistry, risk, and management. CRC Press, Boca Raton, pp 25–40
Yin N, Yang G, Zhong Z, Xing W (2011) Separation of ammonium salts from coking wastewater with nanofiltration combined with diafiltration. Desalination 268:233–237
Young TC, Zhao X, Theis TL (2006) In: Dzombak DA, Ghosh RS, Wong-Chong GM (eds) Cyanide in water and soil: chemistry, risk, and management. CRC Press, Boca Raton, pp 171–190
Yu X, Zhou P, Xishi Z, Liu Y (2005) Cyanide removal y Chinese vegetation—quantification of the Michaelis–Menten kinetics. Environ Sci Pollut Res 12:221–222
Zopfi J, Ferdelman T, Jørgensen BB, Teske A, Thamdrup B (2001) Influence of water column dynamics on sulfide oxidation and other major biogeochemical processes in the chemocline of the stratified Mariager Fjord (Denmark). Mar Chem 74:29–51
Zopfi J, Ferdelman TG, Fossing H (2004) Distribution and fate of sulfur intermediates—sulfite, tetrathionate, thiosulfate, and elemental sulfur—in marine sediments. Geol Soc Am Spec Pap 379:97–116
Acknowledgements
This work was supported by the Marie Curie Actions CIG PCIG10-GA-2011-303740 (ThioCyAnOx) Grant. We are grateful to Ido Ben Laish for help with establishing experimental protocols for analyses of cyanide and sulfur species. The authors would like to thank Valeria Boyko for assistance with experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kurashova, I., Kamyshny, A. Kinetics of Thiocyanate Formation by Reaction of Cyanide and Its Iron Complexes with Thiosulfate. Aquat Geochem 25, 219–236 (2019). https://doi.org/10.1007/s10498-019-09361-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10498-019-09361-y