Abstract
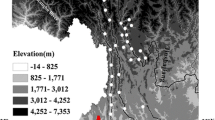
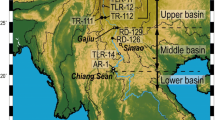
We investigated the dissolved major elements, \( {}^{87}{\text{Sr/}}{}^{86}{\text{Sr}},\;\delta {}^{34}{\text{S}}_{{\text{SO}}_{\text{4}} } ,\;{\text{and}}\;\delta {}^{18}{\text{O}}_{{\text{SO}}_{\text{4}} } \) composition of the Min Jiang, a headwater tributary of the Chang Jiang (Yangtze River). A forward calculation method was applied to quantify the relative contribution to the dissolved load from rain, evaporite, carbonate, and silicate reservoirs. Input from carbonate weathering dominated the major element composition (58–93%) and that from silicate weathering ranged from 2 to 18% in unperturbed Min Jiang watersheds. Most samples were supersaturated with respect to calcite, and the CO2 partial pressures were similar to or up to ∼5 times higher than atmospheric levels. The Sr concentrations in our samples were low (1.3–2.5 μM) with isotopic composition ranging from 0.7108 to 0.7127, suggesting some contribution from felsic silicates. The Si/(Na* + K) ratios ranged from 0.5 to 2.5, which indicate low to moderate silicate weathering intensity. The \( \delta {}^{34}{\text{S}}_{{\text{SO}}_{\text{4}} } \;{\text{and}}\;\delta {}^{18}{\text{O}}_{{\text{SO}}_{\text{4}} } \) for five select samples showed that the source of dissolved sulfate was combustion of locally consumed coal. The silicate weathering rates were 23–181 × 103 mol/km2/year, and the CO2 consumption rates were 31–246 × 103 mol/km2/year, which are moderate on a global basis. Upon testing various climatic and geomorphic factors for correlation with the CO2 consumption rate, the best correlation coefficients found were with water temperature (r 2 = 0.284, p = 0.009), water discharge (r 2 = 0.253, p = 0.014), and relief (r 2 = 0.230, p = 0.019).








Similar content being viewed by others
References
Aas W, Shao M, Jin L, Larssen T, Zhao D, Xiang R, Zhang J, Xiao J, Duan L (2007) Air concentrations and wet deposition of major inorganic ions at five non-urban sites in China, 2001–2003. Atmos Environ 41:1706–1716
Andrews JE, Brimblecombe P, Jickells TD, Liss PS (1996) An introduction to environmental chemistry. Blackwell Science, Oxford
Berner EK, Berner RA (1996) Global environment: water, air, and geochemical cycles. Prentice-Hall, Englewood Cliffs
Bethke CM (2002) The Geochemist’s Workbench 4.0. University of Illinois, USA
Bottrell SH, Tranter M (2002) Sulphide oxidation under partially anoxic conditions at the bed of the Haut Glacier d’Arolla, Switzerland. Hydrol Process 16:2363–2368
Burchfiel BC, Chen Z, Liu Y, Royden LH (1995) Tectonics of the Longmen Shan and adjacent regions, Central China. Int Geol Rev 37:661–735
Bureau of Geology, Mineral Resources of Sichuan Province (1991) Regional geology of Sichuan Province. Geological Publishing House, Beijing
Burke WH, Denison RE, Hetherington EA, Koepnick RB, Nelson HF, Otto JB (1982) Variation of seawater 87Sr/86Sr throughout phanerozoic time. Geology 10(10):516–519
Chen JS, Chu XL (1988) Sulfur isotope composition of Triassic marine sulfates of south China. Chem Geol 72:155–161
Chen J, Wang F, Xia X, Zhang L. (2002) Major element chemistry of the Changjiang (Yangtze River). Chem Geol 187:231–255
Chinese Academy of Sciences Comprehensive Research Team (1985) Hydrologic geography for the western Sichuan and northern Yunnan area. Sciences Publishing House, Beijing
Commision on Annals of Sichuan Province (1998) Annals of Sichuan Province (geology part). Science and Technological Publishing House of Sichuan Province, Chengdu
Dalai TK, Krishnaswami S, Sarin MM (2002) Major ion chemistry in the headwaters of the Yamuna river system: chemical weathering, its temperature dependence and CO2 consumption in the Himalaya. Geochim Cosmochim Acta 66(19):3397–3416
Fekete BM, Vörösmarty CJ, Grabs W (2002) High resolution fields of global runoff combining observed river discharge and simulated water balances. Global Biogeochem Cycles 16(3):1042. doi:10.1029/1999GB001254
Galy A, France-Lanord C (1999) Weathering processes in the Ganges-Brahmaputra basin and the riverine alkalinity budget. Chem Geol 159(1–4):31–60
Goldberg T, Poulton SW, Strauss H (2005) Sulphur and oxygen isotope signatures of late Neoproterozoic to early Cambrian sulphate, Yangtze Platform, China: Diagenetic constraints and seawater evolution. Precambrian Res 137:223–241
Hearn P, Hare T, Schruben P, Sherrill D, Lamar C, Tsushima P (2001) Global GIS database: digital atlas of South Asia. U.S. Geological Survey
Hoefs J (1997) Stable isotope geochemistry, 4th edn. Springer-Verlag, Berlin
Hong YL, Kim G (2005) Measurement of cosmogenic 35S activity in rainwater and lake water. Anal Chem 77:3390–3393
Huh Y, Edmond JM (1999) The fluvial geochemistry of the rivers of Eastern Siberia: III. Tributaries of the Lena and Anabar draining the basement terrain of the Siberian Craton and the Trans-Baikal Highlands. Geochim Cosmochim Acta 63:967–987
Huh Y, Tsoi MY, Zaitsev A, Edmond JM (1998a) The fluvial geochemistry of the rivers of Eastern Siberia: I. Tributaries of the Lena River draining the sedimentary platform of the Siberian Craton. Geochim Cosmochim Acta 62(10):1657–1676
Huh Y, Panteleyev G., Babich D, Zaitsev A, Edmond JM (1998b) The fluvial geochemistry of the rivers of Eastern Siberia: II. Tributaries of the Lena, Omoloy, Yana, Indigirka, Kolyma, and Anadyr draining the collisional/accretionary zone of the Verkhoyansk and Cherskiy ranges. Geochim Cosmochim Acta 62(12):2053–2075
IAEA/WMO (2004) Global network of isotopes in precipitation. The GNIP database. http://isohis.iaea.org. Cited 22 July 2007
Karim A, Veizer J (2000) Weathering processes in the Indus River Basin: implications from riverine carbon, sulfur, oxygen, and strontium isotopes. Chem Geol 170(1–4):153–177
Kurz MD, Kammer DP (1991) Isotopic evolution of Mauna Loa volcano. Earth Planet Sci Lett 103:257–269
Leemans R, Cramer W (1991) The II ASA database for mean monthly values of temperature, precipitation and cloudiness on a global terrestrial grid. II ASA Research Report RR-91-18. International Institute of Applied Systems Analyses
Lerman A, Wu L, Mackenzie FT (2007) CO2 and H2SO4 consumption in weathering and material transport to the ocean, and their role in the global carbon balance. Mar Chem 106:326–350
Li XB, Huang ZL, Li WB, Zhang ZL, Yan ZF (2006a) Sulfur isotopic compositions of the Huize super-large Pb-Zn deposit, Yunnan Province, China: implications for the source of sulfur in the ore-forming fluids. J Geochem Explor 89:227–230
Li XD, Masuda H, Kusakabe M, Yanagisawa F, Zeng HA (2006b) Degradation of groundwater quality due to anthropogenic sulfur and nitrogen contamination in the Sichuan Basin, China. Geochem J 40:309–332
Lloyd RM (1968) Oxygen isotope behaviour in the sulfate-water system. J Geophys Res 73:6099–6110
Map Publishing House of China (1998) Atlas of the Chinese natural geography plates, Beijing
Meybeck M (1979) Concentrations des eaux fluviales en éléments majeurs et apports en solution aux océans. Rev Géol Dyn Géogr Phys 21:215–246
Moon S, Huh Y, Qin J, van Pho N (2007) Chemical weathering in the Hong (Red) River basin: rates of silicate weathering and their controlling factors. Geochim Cosmochim Acta 71(6):1411–1430
Palmer MR, Hevalci C, Fallick AE (2004) Sulfur, sulfate oxygen and strontium isotope composition of Cenozoic Turkish evaporites. Chem Geol 209:341–356
Pan G.T, Cheng ZL, Li XZ, Yan YJ, Xu XS, Xu Q, Jiang XS, Wu YL, Luo JL, Zu TX, Pen YM (1997) Geological-tectonic evolution in the eastern Tethys. Geological Publishing House, Beijing
Qin J, Huh Y, Edmond JM, Du G., Ran J (2006) Chemical and physical weathering in the Min Jiang, a headwater tributary of the Yangtze River. Chem Geol 227(1–2):53–69
Raymo ME, Ruddiman WF (1992) Tectonic forcing of late Cenozoic climate. Nature 359:117–122
Richey JE (2004) Emission of CO2 from riverine systems. In: Steffan E (ed) Global change and the Earth system: a planet under pressure. Springer-Verlag, Berlin, New York, pp 172–173
Smith IE, Compston W (1982) Strontium isotopes in Cenozoic volcanic rocks from southeastern Papua New Guinea. Lithos 15:199–206
Stallard RF, Edmond JM (1987) Geochemistry of the Amazon 3. Weathering chemistry and limits to dissolved inputs. J Geophys Res 92(C8):8293–8302
Toran L, Harris RF (1989) Interpretation of sulfur and oxygen isotopes in biological and abiological sulphide oxidation. Geochim Cosmochim Acta 53:2341–2348
Wanninkhof R, Mulholland PJ, Elwood JW (1990) Gas exchange rates for a first-order stream determined with deliberate and natural tracers. Water Resour Res 26(7):1621–1630
Wu L, Huh Y, Qin J, Du G., van der Lee S (2005) Chemical weathering in the Upper Huang He (Yellow River) draining the eastern Qinghai-Tibet Plateau. Geochim Cosmochim Acta 69(22):5279–5294
Xiao L, Zhang HF, Clemens JD, Wang QW, Kan ZZ, Wang KM, Ni PZ, Liu XM (2007) Late Triassic granitoids of the eastern margin of the Tibetan Plateau: geochronology, petrogenesis and implications for tectonic evolution. Lithos 96:436–452
Zhang DD, Peart M, Jim CY, He YQ, Li BS, Chen JA (2003) Precipitation chemistry of Lhasa and other remote towns, Tibet. Atmos Environ 37:231–240
Acknowledgment
Financial support was provided by the US NSF EAR0134966 and KOSEF grant No. R01-2006-000-10019-0 to Y. Huh and Brain Korea 21 Graduate Student Fellowships to S. Moon, H. Noh, and J. Yoon.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoon, J., Huh, Y., Lee, I. et al. Weathering Processes in the Min Jiang: Major Elements, \( {}^{87}{\text{Sr/}}{}^{86}{\text{Sr}},\;\delta {}^{34}{\text{S}}_{{\text{SO}}_{\text{4}} } ,\;{\text{and}}\;\delta {}^{18}{\text{O}}_{{\text{SO}}_{\text{4}} } \) . Aquat Geochem 14, 147–170 (2008). https://doi.org/10.1007/s10498-008-9030-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10498-008-9030-7