Abstract
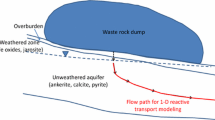
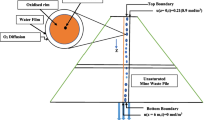
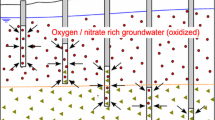
The reactive transport modeling of a complicated suite of reactions apparent in the aquifer during the application of N-containing fertilizers is reported. The unconfined sandy aquifer can be subdivided into an oxic zone which contains groundwater with oxygen and nitrate and an anoxic zone characterized by elevated iron and sulfate concentrations in groundwater. Oxygen and nitrate are being reduced by pyrite and organic matter that commonly apparent in the aquifer. The oxidation of pyrite is modeled using the local equilibrium approach, whereas decomposition of organic matter, with the adoption of kinetic approach. The system is buffered by dissolution of aluminum and iron oxides. The modeling process is a two-step procedure. First, the processes are modeled in the one-dimensional (1D) column using PHREEQC code. Subsequently, the calibrated and verified data were copied and used in two-dimensional (2D) PHAST model. Prior to the performance of reactive transport modeling operations with PHAST, a reliable flow model was executed. Finally, predictions are made for the distribution of water chemistry for the year 2008. Model predicts that sulfate derived from the ongoing pyrite oxidation is reduced by the dissolved organic carbon at the higher depth and forms pyrite by the reaction with iron. The results of this study highlight the importance of understanding the interplay between the transport and chemical reactions that occur during the input of nitrate to the aquifer. Reactive transport modeling incorporating the use of a newly developed code PHAST have proved to be a powerful tool for analyzing and quantifying such interactions.







Similar content being viewed by others
References
Appelo CAJ, Postma D (2005) Geochemistry, groundwater and pollution, 2nd edn. AA Balkema, Rotterdam. 649 pp
Bahr JM, Rubin J (1987) Direct comparison of kinetic and local equilibrium formulations for solute transport affected by surface reactions. Water Resour Res 23:438–452
Engesgaard P, Jensen H (1990) Flow and transport modeling, Rabis Creek. Forsk. Fra Miljøstyrelsen B13. Environmental Protection Agency, Copenhagen
Engesgaard P, Kipp KL (1992) A geochemical transport model for redox-controlled movement of mineral fronts in groundwater flow systems: a case of nitrate removal by oxidation of pyrite. Water Resour Res 28:2829–2843
Engesgaard P, Jensen KH, Molson J, Frind E, Olsen H (1996) Large-scale dispersion in a sandy aquifer: simulation of subsurface transport of environmental tritium. Water Resour Res 32:3253–3266
Engesgaard P, Højberg P, Hinsby K, Jensen KH, Laier T, Larsen F, Busenberg E, Plummer LN (2004) Transport and time lag of chlorofluorocarbon gases in the unsaturated zone, Rabis Creek, Denmark. Vadose Zone J 3:1249–1261
Hinsby K, Højberg P, Engesgaard P, Jensen KH, Larsen F, Plummer LN, Busenberg E (2007) Transport and degradation of chlorofluorocarbons (CFCs) in the pyritic Rabis Creek aquifer, Denmark. Water Resour Res 43, W10423, doi:10.1029/2006WR005854
Jakobsen R, Cold L (2007) Geochemistry of the sulfate reduction-methanogenesis transition zone in an anoxic aquifer—a partial equilibrium interpretation using 2D reactive transport modeling. Geochim Cosmochim Acta 71:1949–1966
Jakobsen R, Postma D (1999) Redox zoning, rates of sulfate reduction and interactions with Fe-reduction and methanogenesis in a shallow sandy aquifer, Rømø, Denmark. Geochim Cosmochim Acta 63:137–151
Keating EH, Bahr JM (1998) Reactive transport modeling of redox geochemistry: approaches to chemical disequilibrium and reaction rate estimation at a site in northern Wisconsin. Water Resour Res 34:3573–3584
Knapp RB (1989) Spatial and temporal scales of local equilibrium in dynamic fluid-rock systems. Geochim Cosmochim Acta 53:1955–1964
Olsen H, Ploug C, Nielsen U, Sørensen K (1993) Reservoir characterization applying high-resolution seismic profiling. Rabis Creek, Denmark. Ground Water 31:84–90
Parkhurst DL, Appelo CAJ (1999) User’s Guide to PHREEQC (Version 2)—A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. Water-Resources Investigations Report 99-4259. U.S. Geological Survey, 312 pp
Parkhurst DL, Kipp KL, Engesgaard P, Charlton SR (2004) PHAST—A program for simulating ground-water flow, solute transport, and multicomponent geochemical reactions. Techniques and Methods 6-A8, U.S. Geological Survey, 154 pp
Postma D, Boesen C, Kristansen H, Larsen F (1991) Nitrate reduction in an unconfined sandy aquifer: water chemistry, reduction processes and geochemical modeling. Water Resour Res 27:2027–2045
Robertson WD, Russell BM, Cherry JA (1996) Attenuation of nitrate in aquitard sediments of southern Ontario. J Hydrol 180:267–281
Tesoriero AJ, Liebscher H, Cox SE (2000) Mechanism and rate of nitrification in an agricultural watershed: Electron and mass balance along groundwater flowpaths. Water Resour Res 36:1545–1559
Acknowledgments
I would like to thank the anonymous reviewers and associate editor for their valuable comments and helpful suggestions. Acknowledgements are also due to the Professors Dieke Postma and Andrzej Kowalczyk for their friendly remarks on the first draft of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miotliński, K. Coupled Reactive Transport Modeling of Redox Processes in a Nitrate-Polluted Sandy Aquifer. Aquat Geochem 14, 117–131 (2008). https://doi.org/10.1007/s10498-008-9028-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10498-008-9028-1