Abstract
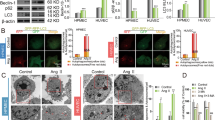
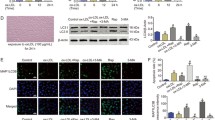
Endothelial apoptosis caused by activation of renin-angiotensin system (RAS) plays a vital part in the occurrence and progress of hypertension. Angiotensin-(1–9) (Ang-(1–9)) is a peptide of the counter-regulatory non-classical RAS with anti-hypertensive effects in vascular endothelial cells (ECs). However, the mechanism of action remains unclear. Considering that the endothelial apoptosis was closely related to endoplasmic reticulum stress (ERS) and mitochondrial function. Herein, we aimed to elucidate the effects of Ang-(1–9) on endothelial apoptosis and the underlying molecular mechanism in angiotensin II (Ang II) induced hypertension. In human umbilical vascular endothelial cells (HUVECs), we observed Ang-(1–9) inhibited Ang II-induced ERS associated endothelial apoptosis. Mechanically, Ang-(1–9) inhibited endothelial apoptosis by blocking CNPY2/PERK mediated CaMKII/Drp1-dependent mitochondrial fission and eIF2α/CHOP signal. Consistent with above effects in HUVECs, in Ang II-induced hypertensive mice, we found administration of exogenous Ang-(1–9) attenuated endothelial apoptosis and arterial blood pressure, which were mediated by CNPY2/PERK signaling pathway. Our study indicated Ang-(1–9) inhibited Ang II-induced hypertension through CNPY2/PERK pathway. These findings may provide new insights for prevention and treatment of hypertension in future.








Similar content being viewed by others
Data availability
All data that support the current study findings are available from the corresponding author on reasonable request.
Change history
20 July 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10495-023-01876-8
Abbreviations
- Ang-(1–9):
-
Angiotensin-(1–9)
- RAS:
-
Renin-angiotensin system
- Ang II:
-
Angiotensin II
- HUVECs:
-
Human umbilical vascular endothelial cells
- ERS:
-
Endoplasmic reticulum stress
- ECs:
-
Endothelial cells
- CNPY2:
-
Canopy FGF signaling regulator 2
- PERK:
-
Protein kinase R-like endoplasmic reticulum kinase
- eIF2α:
-
Eukaryotic initiation factor 2α
- ATF4:
-
Activating transcription factor 4
- CHOP:
-
C/EBP-homologous protein
- Drp1:
-
Dynamin-related protein 1
- CaMKII:
-
Ca2+/calmodulin-dependent protein kinase II
- UPR:
-
Unfolded protein response
- ACE 2:
-
Angiotensin converting enzyme type 2
- ATCC:
-
American type culture collection
- DMEM:
-
Dulbecco’s modified eagle medium
- FBS:
-
Fetal bovine serum
- ROS:
-
Reactive oxygen species
- SD:
-
Standard deviation
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- BAX:
-
BCL2-Associated X
- BCL-2:
-
B-cell lymphoma-2
- eNOS:
-
Endothelin nitric oxide synthase
References
Schwalm JD, McCready T, Lopez-Jaramillo P, Yusoff K, Attaran A, Lamelas P, Camacho PA, Majid F, Bangdiwala SI, Thabane L, Islam S, McKee M, Yusuf S (2019) A community-based comprehensive intervention to reduce cardiovascular risk in hypertension (HOPE 4): a cluster-randomised controlled trial. Lancet 394:1231–1242. https://doi.org/10.1016/S0140-6736(19)31949-X
Rajendran P, Rengarajan T, Thangavel J, Nishigaki Y, Sakthisekaran D, Sethi G, Nishigaki I (2013) The vascular endothelium and human diseases. Int J Biol Sci 9:1057–1069. https://doi.org/10.7150/ijbs.7502
Li XC, Zhang J, Zhuo JL (2017) The vasoprotective axes of the renin-angiotensin system: physiological relevance and therapeutic implications in cardiovascular, hypertensive and kidney diseases. Pharmacol Res 125:21–38. https://doi.org/10.1016/j.phrs.2017.06.005
Forrester SJ, Booz GW, Sigmund CD, Coffman TM, Kawai T, Rizzo V, Scalia R, Eguchi S (2018) Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol Rev 98:1627–1738. https://doi.org/10.1152/physrev.00038.2017
Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S (2000) A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res 87:E1-9. https://doi.org/10.1161/01.res.87.5.e1
Flores-Munoz M, Work LM, Douglas K, Denby L, Dominiczak AF, Graham D, Nicklin SA (2012) Angiotensin-(1–9) attenuates cardiac fibrosis in the stroke-prone spontaneously hypertensive rat via the angiotensin type 2 receptor. Hypertension 59:300–307. https://doi.org/10.1161/hypertensionaha.111.177485
Ocaranza MP, Moya J, Barrientos V, Alzamora R, Hevia D, Morales C, Pinto M, Escudero N, García L, Novoa U, Ayala P, Díaz-Araya G, Godoy I, Chiong M, Lavandero S, Jalil JE, Michea L (2014) Angiotensin-(1–9) reverses experimental hypertension and cardiovascular damage by inhibition of the angiotensin converting enzyme/Ang II axis. J Hypertens 32:771–783. https://doi.org/10.1097/hjh.0000000000000094
Gonzalez L, Novoa U, Moya J, Gabrielli L, Jalil JE, Garcia L, Chiong M, Lavandero S, Ocaranza MP (2018) Angiotensin-(1–9) reduces cardiovascular and renal inflammation in experimental renin-independent hypertension. Biochem Pharmacol 156:357–370. https://doi.org/10.1016/j.bcp.2018.08.045
Sotomayor-Flores C, Rivera-Mejias P, Vasquez-Trincado C, Lopez-Crisosto C, Morales PE, Pennanen C, Polakovicova I, Aliaga-Tobar V, Garcia L, Roa JC, Rothermel BA, Maracaja-Coutinho V, Ho-Xuan H, Meister G, Chiong M, Ocaranza MP, Corvalan AH, Parra V, Lavandero S (2020) Angiotensin-(1–9) prevents cardiomyocyte hypertrophy by controlling mitochondrial dynamics via miR-129-3p/PKIA pathway. Cell Death Differ 27:2586–2604. https://doi.org/10.1038/s41418-020-0522-3
Pober JS, Min W, Bradley JR (2009) Mechanisms of endothelial dysfunction, injury, and death. Annu Rev Pathol 4:71–95. https://doi.org/10.1146/annurev.pathol.4.110807.092155
Battson ML, Lee DM, Gentile CL (2017) Endoplasmic reticulum stress and the development of endothelial dysfunction. Am J Physiol Heart Circ Physiol 312:H355–H367. https://doi.org/10.1152/ajpheart.00437.2016
Barabutis N (2021) Unfolded protein response: a regulator of the endothelial barrier. Endocr Metab Sci. https://doi.org/10.1016/j.endmts.2021.100092
Han S, Bal NB, Sadi G, Usanmaz SE, Tuglu MM, Uludag MO, Demirel-Yilmaz E (2019) Inhibition of endoplasmic reticulum stress protected DOCA-salt hypertension-induced vascular dysfunction. Vascul Pharmacol 113:38–46. https://doi.org/10.1016/j.vph.2018.11.004
Hong F, Liu B, Wu BX, Morreall J, Roth B, Davies C, Sun S, Diehl JA, Li Z (2017) CNPY2 is a key initiator of the PERK-CHOP pathway of the unfolded protein response. Nat Struct Mol Biol 24:834–839. https://doi.org/10.1038/nsmb.3458
Roe ND, Ren J (2013) Oxidative activation of Ca(2+)/calmodulin-activated kinase II mediates ER stress-induced cardiac dysfunction and apoptosis. Am J Physiol Heart Circ Physiol 304:H828-839. https://doi.org/10.1152/ajpheart.00752.2012
Zhong Y, Jin C, Han J, Zhu J, Liu Q, Sun D, Xia X, Peng X (2021) Inhibition of ER stress attenuates kidney injury and apoptosis induced by 3-MCPD via regulating mitochondrial fission/fusion and Ca2+ homeostasis. Cell Biol Toxicol 37:795–809. https://doi.org/10.1007/s10565-021-09589-x
Hu J, Zhang Y, Jiang X, Zhang H, Gao Z, Li Y, Fu R, Li L, Li J, Cui H, Gao N (2019) ROS-mediated activation and mitochondrial translocation of CaMKII contributes to Drp1-dependent mitochondrial fission and apoptosis in triple-negative breast cancer cells by isorhamnetin and chloroquine. J Exp Clin Cancer Res 38:225. https://doi.org/10.1186/s13046-019-1201-4
Wakisaka Y, Chu Y, Miller JD, Rosenberg GA, Heistad DD (2010) Spontaneous intracerebral hemorrhage during acute and chronic hypertension in mice. J Cereb Blood Flow Metab 30:56–69. https://doi.org/10.1038/jcbfm.2009.183
Norambuena-Soto I, Ocaranza MP, Cancino-Arenas N, Sanhueza-Olivares F, Villar-Fincheira P, Leiva-Navarrete S, Mancilla-Medina C, Moya J, Novoa U, Jalil JE, Castro PF, Lavandero S, Chiong M (2020) Angiotensin-(1–9) prevents vascular remodeling by decreasing vascular smooth muscle cell dedifferentiation through a FoxO1-dependent mechanism. Biochem Pharmacol 180:114190. https://doi.org/10.1016/j.bcp.2020.114190
Huang L, Wang A, Hao Y, Li W, Liu C, Yang Z, Zheng F, Zhou MS (2018) Macrophage depletion lowered blood pressure and attenuated hypertensive renal injury and fibrosis. Front Physiol 9:473. https://doi.org/10.3389/fphys.2018.00473
Liang CC, Park AY, Guan JL (2007) In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2:329–333. https://doi.org/10.1038/nprot.2007.30
Cui M, Cai Z, Chu S, Sun Z, Wang X, Hu L, Yi J, Shen L, He B (2016) Orphan nuclear receptor nur77 inhibits angiotensin II-induced vascular remodeling via downregulation of beta-catenin. Hypertension 67:153–162. https://doi.org/10.1161/HYPERTENSIONAHA.115.06114
Sepúlveda-Fragoso V, Alexandre-Santos B, Salles ACP, Proença AB, de Paula Alves AP, Vázquez-Carrera M, Nóbrega ACL, Frantz EDC, Magliano DC (2021) Crosstalk between the renin-angiotensin system and the endoplasmic reticulum stress in the cardiovascular system: lessons learned so far. Life Sci 284:119919. https://doi.org/10.1016/j.lfs.2021.119919
Takemoto-Kimura S, Suzuki K, Horigane SI, Kamijo S, Inoue M, Sakamoto M, Fujii H, Bito H (2017) Calmodulin kinases: essential regulators in health and disease. J Neurochem 141:808–818. https://doi.org/10.1111/jnc.14020
Jin L, Piao ZH, Liu CP, Sun S, Liu B, Kim GR, Choi SY, Ryu Y, Kee HJ, Jeong MH (2018) Gallic acid attenuates calcium calmodulin-dependent kinase II-induced apoptosis in spontaneously hypertensive rats. J Cell Mol Med 22:1517–1526. https://doi.org/10.1111/jcmm.13419
Gkaliagkousi E, Gavriilaki E, Triantafyllou A, Douma S (2015) Clinical significance of endothelial dysfunction in essential hypertension. Curr Hypertens Rep 17:85. https://doi.org/10.1007/s11906-015-0596-3
Wang Q, Zhang M, Ding Y, Wang Q, Zhang W, Song P, Zou MH (2014) Activation of NAD(P)H oxidase by tryptophan-derived 3-hydroxykynurenine accelerates endothelial apoptosis and dysfunction in vivo. Circ Res 114:480–492. https://doi.org/10.1161/circresaha.114.302113
Schröder M, Kaufman RJ (2005) The mammalian unfolded protein response. Annu Rev Biochem 74:739–789. https://doi.org/10.1146/annurev.biochem.73.011303.074134
Karagöz GE, Aragón T, Acosta-Alvear D (2019) Recent advances in signal integration mechanisms in the unfolded protein response. Research. https://doi.org/10.12688/f1000research.19848.1
Jiang S, Shui Y, Cui Y, Tang C, Wang X, Qiu X, Hu W, Fei L, Li Y, Zhang S, Zhao L, Xu N, Dong F, Ren X, Liu R, Persson PB, Patzak A, Lai EY, Wei Q, Zheng Z (2021) Gut microbiota dependent trimethylamine N-oxide aggravates angiotensin II-induced hypertension. Redox Biol 46:102115. https://doi.org/10.1016/j.redox.2021.102115
Timmins JM, Ozcan L, Seimon TA, Li G, Malagelada C, Backs J, Backs T, Bassel-Duby R, Olson EN, Anderson ME, Tabas I (2009) Calcium/calmodulin-dependent protein kinase II links ER stress with fas and mitochondrial apoptosis pathways. J Clin Invest 119:2925–2941. https://doi.org/10.1172/jci38857
Zhao S, Yuan C, Tuo X, Zhou C, Zhao Q, Shen T (2021) MCLR induces dysregulation of calcium homeostasis and endoplasmic reticulum stress resulting in apoptosis in Sertoli cells. Chemosphere 263:127868. https://doi.org/10.1016/j.chemosphere.2020.127868
Sun L, Liu YL, Ye F, Xie JW, Zeng JW, Qin L, Xue J, Wang YT, Guo KM, Ma MM, Tang YB, Li XY, Gao M (2019) Free fatty acid-induced HO activates TRPM2 to aggravate endothelial insulin resistance via Ca-dependent PERK/ATF4/TRB3 cascade in obese mice. Free Radic Biol Med 143:288–299. https://doi.org/10.1016/j.freeradbiomed.2019.08.018
Uchikado Y, Ikeda Y, Ohishi M (2022) Current understanding of the pivotal role of mitochondrial dynamics in cardiovascular diseases and senescence. Front Cardiovasc Med 9:905072. https://doi.org/10.3389/fcvm.2022.905072
Jin JY, Wei XX, Zhi XL, Wang XH, Meng D (2021) Drp1-dependent mitochondrial fission in cardiovascular disease. Acta Pharmacol Sin 42:655–664. https://doi.org/10.1038/s41401-020-00518-y
Deng Y, Li S, Chen Z, Wang W, Geng B, Cai J (2021) Mdivi-1, a mitochondrial fission inhibitor, reduces angiotensin-II- induced hypertension by mediating VSMC phenotypic switch. Biomed Pharmacother 140:111689. https://doi.org/10.1016/j.biopha.2021.111689
Hayashida R, Kondo K, Morita S, Unno K, Shintani S, Shimizu Y, Calvert JW, Shibata R, Murohara T (2017) Diallyl trisulfide augments ischemia-induced angiogenesis via an endothelial nitric oxide synthase-dependent mechanism. Circ J 81:870–878. https://doi.org/10.1253/circj.CJ-16-1097
Pan J, Zhang L, Xu S, Cheng X, Yu H, Bao J, Lu R (2018) Induction of apoptosis in human papillary-thyroid-carcinoma BCPAP cells by diallyl trisulfide through activation of the MAPK signaling pathway. J Agric Food Chem 66:5871–5878. https://doi.org/10.1021/acs.jafc.8b02243
Lebeau J, Saunders JM, Moraes VWR, Madhavan A, Madrazo N, Anthony MC, Wiseman RL (2018) The PERK arm of the unfolded protein response regulates mitochondrial morphology during acute endoplasmic reticulum stress. Cell Rep 22:2827–2836. https://doi.org/10.1016/j.celrep.2018.02.055
Almeida LM, Pinho BR, Duchen MR, Oliveira JMA (2022) The PERKs of mitochondria protection during stress: insights for PERK modulation in neurodegenerative and metabolic diseases. Biol Rev Camb Philos Soc Undefined. https://doi.org/10.1111/brv.12860
Hatta K, Guo J, Ludke A, Dhingra S, Singh K, Huang ML, Weisel RD, Li RK (2014) Expression of CNPY2 in mouse tissues: quantification and localization. PLoS ONE 9:e111370. https://doi.org/10.1371/journal.pone.0111370
Guo J, Zhang Y, Mihic A, Li S, Sun Z, Shao Z, Wu J, Weisel RD, Li RK (2015) A secreted protein (Canopy 2, CNPY2) enhances angiogenesis and promotes smooth muscle cell migration and proliferation. Cardiovasc Res 105:383–393. https://doi.org/10.1093/cvr/cvv010
Acknowledgements
This work was supported by the Chinese Natural Science Foundation grants (81800171, 81900275), the Natural Science Foundation of Shanxi Province (201801D221273), the Scientific and Technological Innovation Program of Shanxi Higher Education Institution (201804026, 201804027) and Shanxi Provincial Commission of Health and Family Planning (2017053).
Author information
Authors and Affiliations
Contributions
CLG participated in the conception and design of the work, obtained and analysed data and wrote the manuscript. HML performed the experiments and materials, and wrote the manuscript. BL designed the experiments, and wrote the manuscript. ZYL supervised the work, performed the experiments, analysed data, and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interest
The authors have no relevant financial or non-financial interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, Cl., Liu, Hm., Li, B. et al. Angiotensin-(1–9) prevents angiotensin II-induced endothelial apoptosis through CNPY2/PERK pathway. Apoptosis 28, 379–396 (2023). https://doi.org/10.1007/s10495-022-01793-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-022-01793-2