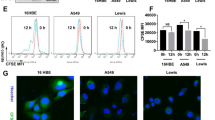
Abstract
1,4-Dihydropyridines (1,4-DHPs) are important as a class of heterocyclic compounds that exhibit wide range of biological actions. Many of its derivatives are already characterized as medicinally important drugs and used worldwide. In this study, we have screened some novel Hantzsch 1,4-DHP compounds using both in silico (QSAR and Pharmacophore) and in vitro (cytotoxic screening). 1,4-DHP showed selective cytotoxicity against five human cancerous cell lines; A375, A549, HeLa, HepG2 and SH-SY5Y but limited effect towards normal skin keratinocyte (HaCaT), lung fibroblast (WL-38) and healthy peripheral blood mononuclear cells. In A375 and HepG2 cells, one of the 1,4-DHP derivative (DHP-8) was found to inhibit cell proliferation, and simultaneously increased the apoptotic population as well as mitochondrial membrane depolarization. Furthermore, the mitochondrial signal was triggered with the activation of cleaved Caspase9, Caspase3 and PARP. The treatment with DHP-8 also increased the expression level of SIRT1, subsequently decreasing the level of pAKTser473 and survivin. Reduced pAKTser473 expression led to decrease the phosphorylated inactive form of GSK3βser9 and as a result, proteasomal degradation of Mcl-1 occurred in both the cell lines. Here, we suggest that the apoptotic effect of DHP-8 in A375 and HepG2 cells was mediated by AKT and survivin pathways through SIRT1 activation. The involvement of DHP-8 in SIRT1 activation was further verified by co-treatment of nicotinamide with DHP-8 in both A375 and HepG2 cells. Overall, this study emphasizes the possible potential and therapeutic role of DHP-8 in skin and liver cancer.












Similar content being viewed by others
References
Hantzsch A (1883) Ueber die synthese pyridinartiger verbindungen aus acetessigäther und aldehydammoniak. Eur J Org Chem 215:1–82. https://doi.org/10.1002/cber.18830160284
Datar PA, Auti PB (2012) Design and synthesis of novel 4-substituted 1,4-dihydropyridine derivatives as hypotensive agents. J Saudi Chem Soc. https://doi.org/10.1016/j.jscs.2012.08.003
Fleckenstein A (1983) History of calcium antagonists. Circ Res 52:I3–I16
Triggle DJ (1990) Calcium,calcium channels, and calcium channel antagonists. Can J Physiol Pharmacol 68:1474–1481
Boer R, Gekeler V (1995) Chemosensitizers in tumor therapy: new compounds promise better efficacy. Drugs Future 20:499–509
Briukhanov VM, Zverev IF, Elkin VI (1994) The effect of calcium antagonists on the development of inflammatory edema in rats (in Russian). Exp Clin Pharmacol 57:47–49
Bahekar S, Shinde D (2002) Synthesis and anti-inflammatory activity of 1-4-dihydropyridines. Acta Pharm 52:281–287
Gullapalli S, Ramarao P (2002) L-type Ca2+ channel modulation by dihydropyridines potentiates κ-opioid receptor agonist induced acute analgesia and inhibits development of tolerance in rats. Neuropharmacology 42:467–475. https://doi.org/10.1016/S0028-3908(01)00200-3
Sandjo LP, Kuete V, Nana F et al (2016) Synthesis and cytotoxicity of 1,4-dihydropyridines and an unexpected 1,3-oxazin-6-one. Helv Chim Acta 99:310–314. https://doi.org/10.1002/hlca.201500265
Razzaghi-Asl N, Miri R, Firuzi O (2016) Assessment of the cytotoxic effect of a series of 1,4-dihydropyridine derivatives against human cancer cells. Iran J Pharm Res 15:413–420
Bhaumik A, Bhongiri B, Devika K et al (2015) Synthetic novel 1, 4-dihydropyridine derivatives act as potential anticancer agent against both human small cell lung DMS 114 cancer cell line and human colon cancer cell line HCC 2998. Am J Pharm Heal Res 3:80–89. https://doi.org/10.17265/2328-2150/2015.02.005
Kumar RS, Idhayadhulla A, Abdul Nasser AJ, Selvin J (2011) Synthesis and anticoagulant activity of a new series of 1,4-dihydropyridine derivatives. Eur J Med Chem 46:804–810. https://doi.org/10.1016/j.ejmech.2010.12.006
Miri R, Javidnia K, Amirghofran Z et al (2011) Cytotoxic effect of some 1,4-dihydropyridine derivatives containing nitroimidazole moiety. Iran J Pharm Res 10:497–503
Sirisha K, Achaiah G, Reddy VM (2010) Facile synthesis and antibacterial, antitubercular, and anticancer activities of novel 1,4-dihydropyridines. Arch Pharm 343:342–352. https://doi.org/10.1002/ardp.200900243
Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414:105–111. https://doi.org/10.1038/35102167
Anand P, Kunnumakara AB, Sundaram C et al (2008) Cancer is a preventable disease that requires major lifestyle changes. Pharm Res 25:2097–2116. https://doi.org/10.1007/s11095-008-9661-9
Zu G, Ji A, Zhou T, Che N (2016) Clinicopathological significance of SIRT1 expression in colorectal cancer: a systematic review and meta analysis. Int J Surg 26:32–37. https://doi.org/10.1016/j.ijsu.2016.01.002
Lim CS (2007) Human SIRT1: a potential biomarker for tumorigenesis? Cell Biol Int 31:636–637. https://doi.org/10.1016/j.cellbi.2006.11.003
Zhang S, Wang XI (2013) SIRT1 is a useful biomarker for high-grade dysplasia and carcinoma in barrett’s esophagus. Ann Clin Lab Sci 43:373–377
Hubbard BP, Sinclair DA (2014) Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol Sci 35:146–154. https://doi.org/10.1016/j.tips.2013.12.004
D’Onofrio N, Vitiello M, Casale R et al (2015) Sirtuins in vascular diseases: emerging roles and therapeutic potential. Biochim Biophys Acta 1852:1311–1322. https://doi.org/10.1016/j.bbadis.2015.03.001
Scuderi C, Stecca C, Bronzuoli R. MR et al (2014) Sirtuin modulators control reactive gliosis in an in vitro model of alzheimer’s disease. Front Pharmacol. https://doi.org/10.3389/fphar.2014.00089
Mellini P, Valente S, Mai A (2015) Sirtuin modulators: an updated patent review (2012–2014). Expert Opin Ther Pat 25:5–15. https://doi.org/10.1517/13543776.2014.982532
Baur JA, Pearson KJ, Price NL et al (2006) Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444:337–342. https://doi.org/10.1038/nature05354
Guarente L, Guarente L (2007) Sirtuins in aging and disease. Cold Spring Harb Symp Quant Biol 72:483–488. https://doi.org/10.1101/sqb.2007.72.024
Wood JG, Rogina B, Lavu S et al (2004) Corrigendum: sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 431:107–107. https://doi.org/10.1038/nature02941
Yang X-J, Seto E (2008) The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nat Rev Mol Cell Biol 9:206–218. https://doi.org/10.1038/nrm2346
Martinez-Redondo P, Vaquero A (2013) The Diversity of histone versus nonhistone Sirtuin substrates. Genes Cancer 4:148–163. https://doi.org/10.1177/1947601913483767
Deng CX (2009) SIRT1, is it a tumor promoter or tumor suppressor? Int J Biol Sci 5:147–152. https://doi.org/10.7150/ijbs.5.147
Song NY, Surh YJ (2012) Janus-faced role of SIRT1 in tumorigenesis. Ann NY Acad Sci 1271:10–19. https://doi.org/10.1111/j.1749-6632.2012.06762.x
Yi J, Luo J (2010) SIRT1 and p53, effect on cancer, senescence and beyond. Biochim Biophys Acta 1804:1684–1689. https://doi.org/10.1016/j.bbapap.2010.05.002
Chen S, Xiao X, Feng X et al (2012) Resveratrol induces Sirt1-dependent apoptosis in 3T3-L1 preadipocytes by activating AMPK and suppressing AKT activity and survivin expression. J Nutr Biochem 23:1100–1112. https://doi.org/10.1016/j.jnutbio.2011.06.003
Altieri DC (2008) Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer 8:61–70. https://doi.org/10.1038/nrc2293
Horvitz HR (1999) Genetic control of programmed cell death in the nematode Caenorhabditis elegans. Cancer Res 59:1701S–1706S. https://doi.org/10.1016/0092-8674(86)90004-8
Cross DA, Alessi DR, Cohen P et al (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785–789. https://doi.org/10.1038/378785a0
Fiol CJ, Mahrenholz AM, Wang Y et al (1987) Formation of protein kinase recognition sites by covalent modification of the substrate. Molecular mechanism for the synergistic action of casein kinase II and glycogen synthase kinase 3. J Biol Chem 262:14042–14048
Embi N, Rylatt DB, Cohen P (1980) Glycogen synthase kinase-3 from rabbit skeletal muscle. Eur J Biochem 107:519–527. https://doi.org/10.1111/j.1432-1033.1980.tb06059.x
Eldar-Finkelman H, Krebs EG (1997) Phosphorylation of insulin receptor substrate 1 by glycogen synthase kinase 3 impairs insulin action. Proc Natl Acad Sci USA 94:9660–9664. https://doi.org/10.1073/pnas.94.18.9660
Yost C, Torres M, Miller JR et al (1996) The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev 10:1443–1454. https://doi.org/10.1101/gad.10.12.1443
Sears R, Nuckolls F, Haura E et al (2000) Multiple ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev 14:2501–2514. https://doi.org/10.1101/gad.836800
Diehl JA, Cheng M, Roussel MF, Sherr CJ (1998) Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev 12:3499–3511. https://doi.org/10.1101/gad.12.22.3499
Welcker M, Singer J, Loeb KR et al (2003) Multisite phosphorylation by Cdk2 and GSK3 controls cyclin E degradation. Mol Cell 12:381–392. https://doi.org/10.1016/S1097-2765(03)00287-9
Viatour P, Dejardin E, Warnier M et al (2004) GSK3-mediated BCL-3 phosphorylation modulates its degradation and its oncogenicity. Mol Cell 16:35–45. https://doi.org/10.1016/j.molcel.2004.09.004
Ghosh S, Saikh F, Das J, Pramanik AK (2013) Hantzsch 1,4-dihydropyridine synthesis in aqueous ethanol by visible light. Tetrahedron Lett 54:58–62. https://doi.org/10.1016/j.tetlet.2012.10.079
Lipinski CA (2004) Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol 1:337–341. https://doi.org/10.1016/j.ddtec.2004.11.007
Martens H, Naes T (1989) Multivariate calibration. Spectrochim Acta A 44:287–321
Ghosh R, Guha D, Bhowmik S, Karmakar S (2013) Antioxidant enzymes and the mechanism of the bystander effect induced by ultraviolet C irradiation of A375 human melanoma cells. Mutat Res 757:83–90. https://doi.org/10.1016/j.mrgentox.2013.06.022
Panda SK, Ravindran B (2013) Isolation of human PBMCs. Bio-Protocol 3:4–6. https://doi.org/10.21769/BioProtoc.323
Ghosh R, Bhowmik S, Guha D (2012) 9-phenyl acridine exhibits antitumour activity by inducing apoptosis in A375 cells. Mol Cell Biochem 361:55–66. https://doi.org/10.1007/s11010-011-1088-7
Ghosh R, Guha D, Bhowmik S (2012) UV released factors induce antioxidant defense in A375 cells. Photochem Photobiol 88:708–716. https://doi.org/10.1111/j.1751-1097.2012.01105.x
Maji S, Samal SK, Pattanaik L et al (2015) Mcl-1 is an important therapeutic target for oral squamous cell carcinomas. Oncotarget 6:16623–16637. https://doi.org/10.18632/oncotarget.3932
Sadhukhan P, Saha S, Sinha K et al (2016) Selective pro-apoptotic activity of novel 3,3′-(aryl/alkyl-methylene)bis(2-hydroxynaphthalene-1,4-dione) derivatives on human cancer cells via the induction reactive oxygen species. PLoS ONE. https://doi.org/10.1371/journal.pone.0158694
Kim YM, Talanian RV, Billiar TR (1997) Nitric oxide inhibits apoptosis by preventing increases in caspase-3-like activity via two distinct mechanisms. J Biol Chem 272:31138–31148. https://doi.org/10.1074/jbc.272.49.31138
Khuda-Bukhsh AR, Biswas R, Mandal SK et al (2011) Thujone-rich fraction of Thuja occidentalis demonstrates major anti-cancer potentials: evidences from in vitro studies on A375 cells. Evidence-Based Complement Altern Med. https://doi.org/10.1093/ecam/neq042
Salido M, Gonzalez JL, Vilches J (2007) Loss of mitochondrial membrane potential is inhibited by bombesin in etoposide-induced apoptosis in PC-3 prostate carcinoma cells. Mol Cancer Ther 6:1292–1299. https://doi.org/10.1158/1535-7163.MCT-06-0681
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101–1108. https://doi.org/10.1038/nprot.2008.73
Dash R, Richards JE, Su ZZ et al (2010) Mechanism by which Mcl-1 regulates cancer-specific apoptosis triggered by mda-7/IL-24, an IL-10-related cytokine. Cancer Res 70:5034–5045. https://doi.org/10.1158/0008-5472.CAN-10-0563
Swarnalatha G, Prasanthi G, Sirisha N, Madhusudhana Chetty C (2011) 1,4-Dihydropyridines: a multtifunctional molecule—a review. Int J ChemTech Res 3:75–89
Foroughinia F, Javidnia K, Amirghofran Z et al (2008) Design and synthesis of new symmetrical derivatives of dihydropyridine containing a pyridyl group on the 3, 5-positions and evaluation of their cytotoxic and multidrug resistance reversal activity. J Pharm Pharmacol 60:1481–1489. https://doi.org/10.1211/jpp/60.11.0009
Cherkasov A, Muratov EN, Fourches D et al (2014) QSAR modeling: where have you been? Where are you going to? J Med Chem 57:4977–5010. https://doi.org/10.1021/jm4004285
Yang S-Y (2010) Pharmacophore modeling and applications in drug discovery: challenges and recent advances. Drug Discov Today 15:444–450. https://doi.org/10.1016/j.drudis.2010.03.013
Marín-Prida J, Pardo Andreu GL, Rossignoli CP et al (2017) The cytotoxic effects of VE-3N, a novel 1,4-dihydropyridine derivative, involve the mitochondrial bioenergetic disruption via uncoupling mechanisms. Toxicol In Vitro 42:21–30. https://doi.org/10.1016/j.tiv.2017.03.011
Dörrie J, Gerauer H, Wachter Y, Zunino SJ (2001) Resveratrol induces extensive apoptosis by depolarizing mitochondrial membranes and activating caspase-9 in acute lymphoblastic leukemia cells. Cancer Res 61:4731–4739
Lee S-H, Meng XW, Flatten KS et al (2013) Phosphatidylserine exposure during apoptosis reflects bidirectional trafficking between plasma membrane and cytoplasm. Cell Death Differ 20:64–76. https://doi.org/10.1038/cdd.2012.93
Riedl SJ, Salvesen GS (2007) The apoptosome: signalling platform of cell death. Nat Rev Mol Cell Biol 8:405–413. https://doi.org/10.1038/nrm2153
Mohamed MF, Darweesh AF, Elwahy AHM, Abdelhamid IA (2016) Synthesis, characterization and antitumor activity of novel tetrapodal 1,4-dihydropyridines: p53 induction, cell cycle arrest and low damage effect on normal cells induced by genotoxic factor H2O2. RSC Adv 6:40900–40910. https://doi.org/10.1039/C6RA04974E
Chaitanya G, Alexander JS, Babu P (2010) PARP-1 cleavage fragments: signatures of cell-death proteases in neurodegeneration. Cell Commun Signal 8:31. https://doi.org/10.1186/1478-811X-8-31
Kaufmann SH, Desnoyers S, Ottaviano Y et al (1993) Specific proteolytic cleavage of Poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res 53:3976–3985. https://doi.org/10.1074/jbc.274.33.22932
Yip KW, Reed JC (2008) Bcl-2 family proteins and cancer. Oncogene 27:6398–6406. https://doi.org/10.1038/onc.2008.307
Pan R, Ruvolo VR, Wei J et al (2015) Inhibition of Mcl-1 with the pan-Bcl-2 family inhibitor (-)BI97D6 overcomes ABT-737 resistance in acute myeloid leukemia. Blood 126:363–372. https://doi.org/10.1182/blood-2014-10-604975
Roberts AW, Seymour JF, Brown JR et al (2012) Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol 30:488–496. https://doi.org/10.1200/JCO.2011.34.7898
Mai A, Valente S, Meade S et al (2009) Study of 1,4-dihydropyridine structural scaffold: discovery of novel sirtuin activators and inhibitors. J Med Chem 52:5496–5504. https://doi.org/10.1021/jm9008289
Valente S, Mellini P, Spallotta F et al (2016) 1,4-Dihydropyridines active on the SIRT1/AMPK pathway ameliorate skin repair and mitochondrial function and exhibit inhibition of proliferation in cancer cells. J Med Chem 59:1471–1491. https://doi.org/10.1021/acs.jmedchem.5b01117
Schug TT, Li X (2011) Sirtuin 1 in lipid metabolism and obesity. Ann Med 43:198–211. https://doi.org/10.3109/07853890.2010.547211
Saunders LR, Verdin E (2007) Sirtuins: critical regulators at the crossroads between cancer and aging. Oncogene 26:5489–5504. https://doi.org/10.1038/sj.onc.1210616
Pfluger PT, Herranz D, Velasco-Miguel S et al (2008) Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci USA 105:9793–9798. https://doi.org/10.1073/pnas.0802917105
Menssen A, Hydbring P, Kapelle K et al (2012) The c-MYC oncoprotein, the NAMPT enzyme, the SIRT1-inhibitor DBC1, and the SIRT1 deacetylase form a positive feedback loop. Proc Natl Acad Sci 109:E187–E196. https://doi.org/10.1073/pnas.1105304109
Roth M, Chen WY (2014) Sorting out functions of sirtuins in cancer. Oncogene 33:1609–1620. https://doi.org/10.1038/onc.2013.120
Smith JJ, Kenney RD, Gagne DJ et al (2009) Small molecule activators of SIRT1 replicate signaling pathways triggered by calorie restriction in vivo. BMC Syst Biol 3:31. https://doi.org/10.1186/1752-0509-3-31
Nogueiras R, Habegger KM, Chaudhary N et al (2012) Sirtuin 1 and Sirtuin 3: physiological modulators of metabolism. Physiol Rev 92:1479–1514. https://doi.org/10.1152/physrev.00022.2011
Venkatasubramanian S, Noh RM, Daga S et al (2016) Effects of the small molecule SIRT1 activator, SRT2104 on arterial stiffness in otherwise healthy cigarette smokers and subjects with type 2 diabetes mellitus. Open Heart 3:e000402. https://doi.org/10.1136/openhrt-2016-000402
Zhang Z, Yang Y, Pang W et al (2010) Effect and underlying mechanism of resveratol on porcine primary preadipocyte apoptosis. Sheng Wu Gong Cheng Xue Bao 26:1042–1049
Park G, Jeong JW, Kim JE (2011) SIRT1 deficiency attenuates MPP+-induced apoptosis in dopaminergic cells. FEBS Lett 585:219–224. https://doi.org/10.1016/j.febslet.2010.11.048
Testa JR, Bellacosa a (2001) AKT plays a central role in tumorigenesis. Proc Natl Acad Sci USA 98:10983–10985. https://doi.org/10.1073/pnas.211430998
Arboleda MJ, Lyons JF, Kabbinavar FF et al (2003) Overexpression of AKT2/protein kinase Bbeta leads to up-regulation of beta1 integrins, increased invasion, and metastasis of human breast and ovarian cancer cells. Cancer Res 63:196–206. https://doi.org/10.1111/J.1464-410X.2004.04574.X
Mukohara T, Kudoh S, Yamauchi S et al (2003) Expression of epidermal growth factor receptor (EGFR) and downstream-activated peptides in surgically excised non-small-cell lung cancer (NSCLC). Lung Cancer 41:123–130. https://doi.org/10.1016/S0169-5002(03)00225-3
Liao Y, Grobholz R, Abel U et al (2003) Increase of AKT/PKB expression correlates with gleason pattern in human prostate cancer. Int J Cancer 107:676–680. https://doi.org/10.1002/ijc.11471
Guha M, Altieri DC (2009) Survivin as a global target of intrinsic tumor suppression networks. Cell Cycle 8:2708–2710. https://doi.org/10.4161/cc.8.17.9457
Kahn M (2014) Can we safely target the WNT pathway? Nat Rev Drug Discov 13:513–532. https://doi.org/10.1038/nrd4233
Firestein R, Blander G, Michan S et al (2008) The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS ONE. https://doi.org/10.1371/journal.pone.0002020
Donmez G, Guarente L (2010) Aging and disease: connections to Sirtuin. Aging Cell 9:285–290. https://doi.org/10.1111/j.1474-9726.2010.00548.x
Ray M, Rai N, Jana K et al (2015) Beta catenin is degraded by both caspase-3 and proteasomal activity during resveratrol-induced apoptosis in hela cells in a GSK3β-independent manner. Indian J Biochem Biophys 52:7–13
Wu D, Pan W (2010) GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci 35:161–168. https://doi.org/10.1016/j.tibs.2009.10.002
Maurer U, Charvet C, Wagman AS et al (2006) Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell 21:749–760. https://doi.org/10.1016/j.molcel.2006.02.009
Ding Q, He X, Hsu J-M et al (2007) Degradation of Mcl-1 by -TrCP mediates glycogen synthase kinase 3-induced tumor suppression and chemosensitization. Mol Cell Biol 27:4006–4017. https://doi.org/10.1128/MCB.00620-06
Peled T, Shoham H, Aschengrau D et al (2012) Nicotinamide, a SIRT1 inhibitor, inhibits differentiation and facilitates expansion of hematopoietic progenitor cells with enhanced bone marrow homing and engraftment. Exp Hematol. https://doi.org/10.1016/j.exphem.2011.12.005
Chong ZZ, Lin SH, Li F, Maiese K (2005) The sirtuin inhibitor nicotinamide enhances neuronal cell survival during acute anoxic injury through AKT, BAD, PARP, and mitochondrial associated “anti-apoptotic” pathways. Curr Neurovasc Res 2:271–285. https://doi.org/10.2174/156720205774322584
Acknowledgements
The authors acknowledge infrastructural support from KU and other instrumental facilities at the Department of Biochemistry & Biophysics, KU funded by DST-FIST, DST-PURSE, UGC-SAP, Govt. of India. The computational work was performed at Bioinformatics Infrastructure Facility (BIF), KU funded by Department of Biotechnology (DBT-India).
Funding
The following authors acknowledge financial support with fellowships: DM - University Scholarship, K.U. (Grant Number M.S.No.Rev/1172 of 2016-17), RB - Research Assistant under BTISNet program at KU funded by Department of Biotechnology, Ministry of Science and Technology (DBT-India) (Grant Number BT/BI/25/001/2006), FS - funded by Council of Scientific and Industrial Research (Grant No. 09/096(0696)/2011-EMR-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have read the journal’s policy and they declare that there is no competing or financial interests associated with the manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10495_2018_1483_MOESM1_ESM.docx
Fig. S1 a, b) A357 and HepG2 cells were treated with different concentrations of DHP-8 and NAM to analyse the induction of apoptosis by Annexin V-7AAD/ PE (Invitrogen) staining through FACS LSRFORTESSA. A typical snapshot of dual parameter dot plot of 7AAD and PE labelled AnnexinV fluorescence (X-axis) and PE (Y-axis) in logarithmic fluorescence intensity are shown. c, d) The bar diagram shows the p-AKT/ AKT ratio for the activation of AKT in A375 and HepG2 cells. All data were the Mean ± SD (n=3) and analyzed by one-way ANOVA. ‘*’ represents the significant difference between the untreated and DHP-8 treated cells (P* < 0.05). (DOCX 2013 KB)
Rights and permissions
About this article
Cite this article
Manna, D., Bhuyan, R., Saikh, F. et al. Novel 1,4-dihydropyridine induces apoptosis in human cancer cells through overexpression of Sirtuin1. Apoptosis 23, 532–553 (2018). https://doi.org/10.1007/s10495-018-1483-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-018-1483-6