Abstract
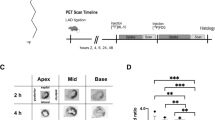
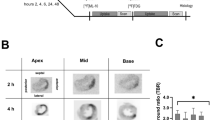
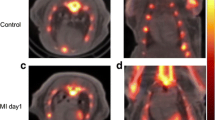
Cardiomyocyte apoptosis has been observed in several cardiovascular diseases and contributes to the subsequent cardiac remodeling processes and progression to heart failure. Consequently, apoptosis imaging is helpful for noninvasively detecting the disease progression and providing treatment guidance. Here, we tested 18F-labeled 2-(5-fluoropentyl)-2-methyl-malonic acid (18F-ML-10) and 18F-labeled 2-(3-fluoropropyl)-2-methyl-malonic acid (18F-ML-8) for apoptosis imaging in rat models of myocardial infarction (MI) and compared them with 18F-fluorodeoxyglucose (18F-FDG). MI was induced in Sprague-Dawley rats by permanent left coronary artery ligation. Procedural success was confirmed by echocardiography and positron emission tomography (PET) imaging with 18F-FDG. In vivo PET imaging with 18F-ML-10 and 18F-ML-8 was performed in the MI models at different time points after operation. Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assays and immunohistochemical analyses were used to evaluate myocardial apoptosis. In vitro cell binding assays were performed to validate 18F-ML-8 binding to apoptotic cardiomyocytes. PET imaging demonstrated high 18F-ML-10 and 18F-ML-8 uptake where 18F-FDG uptake was absent. The focal accumulation of the two tracers was high on days 1 and 3 but was not notable on days 5 and 7 after surgery. The infarct-to-lung uptake ratio was 4.29 ± 0.30 for 18F-ML-10 and 3.51 ± 0.18 for 18F-ML-8 (n = 6, analyzed by averaging the uptake ratios on postoperative days 1 and 3, P < 0.05). The TUNEL results showed that myocardial cell apoptosis was closely related to the focal uptake of the apoptotic tracers in the infarct area. In addition, the apoptosis rates calculated from the TUNEL results were better correlated with 18F-ML-8 uptake than with 18F-ML-10 uptake. Ex vivo cell binding assays demonstrated that 18F-ML-8 accumulated in apoptotic cells but not in necrotic or normal cells. PET imaging using 18F-ML-10 or 18F-ML-8 allows the noninvasive detection of myocardial apoptosis in the early phase. In addition, 18F-ML-8 may be better than 18F-ML-10 for apoptosis imaging. We propose that PET imaging with 18F-ML-10 or 18F-ML-8 combined with 18F-FDG is an alternative for detecting and assessing MI.







Similar content being viewed by others
References
Thygesen K, Alpert JS, Jaffe AS et al (2012) Third universal definition of myocardial infarction. Eur Heart J 33:2551–2567
Kajstura J, Cheng W, Reiss K et al (1996) Apoptotic and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Lab Investig 74:86–107
Zhang Q, Yu N, Yu BT (2018) MicroRNA-298 regulates apoptosis of cardiomyocytes after myocardial infarction. Eur Rev Med Pharmacol Sci 22(2):532–539
Foglio E, Puddighinu G, Germani A et al (2017) HMGB1 inhibits apoptosis following MI and induces autophagy via mTORC1 inhibition. J Cell Physiol 232(5):1135–1143
Lotats A, Carrió I (2003) Non-invasive in vivo imaging of myocardial apoptosis and necrosis. Eur J Nucl Med Mol Imaging 30(4):615–630
Rodríguez M, Lucchesi BR, Schaper J (2002) Apoptosis in myocardial infarction. Ann Med 34(6):470–479
Reshef A, Shirvan A, Wall A et al (2010) Small-molecule biomarkers for clinical PET imaging of apoptosis. J Nucl Med 51:837–840
Sarda-Mantel L, Michel JB, Rouzet F et al (2006) 99mTc-annexin V and 111In-antimyosin antibody uptake in experimental myocardial infarction in rats. Eur J Nucl Med Mol Imaging 33:239–245
Zhao M, Zhu X, Ji S et al (2006) 99mTc-labeled C2A domain of synaptotagmin I as a target-specific molecular probe for noninvasive imaging of acute myocardial infarction. J Nucl Med 47:1367–1374
Cohen A, Shirvan A, Levin G et al (2009) From the Gla domain to a novel small-molecule detector of apoptosis. Cell Res 19:625–637
Allen AM, Ben-Ami M, Reshef A et al (2012) Assessment of response of brain metastases to radiotherapy by PET imaging of apoptosis with 18F-ML-10. Eur J Nucl Med Mol Imaging 39:1400–1408
Oborski MJ, Laymon CM, Lieberman FS et al (2014) First use of 18F-labeled ML-10 PET to assess apoptosis change in a newly diagnosed glioblastoma multiforme patient before and early after therapy. Brain Behav 4:312–315
Yao S, Hu K, Tang G et al (2015) Molecular PET Imaging of cyclophosphamide induced apoptosis with 18F-ML-8. Biomed Res Int 2015:1–10
Patten RD, Aronovitz MJ, Deras-Mejia L et al (1998) Ventricular remodeling in a mouse model of myocardial infarction. Am J Physiol 274:H1812–H1820
Gao XM, Dart AM, Dewar E et al (2000) Serial echocardiographic assessment of left ventricular dimensions and function after myocardial infarction in mice. Cardiovasc Res 45:330–338
Ahn D, Cheng L, Moon C et al (2004) Induction of myocardial infarcts of a predictable size and location by branch pattern probability-assisted coronary ligation in C57BL/6 mice. Am J Physiol Heart Circ Physiol 286:H1201–H1207
Wu JC, Chen IY, Wang Y et al (2004) Molecular imaging of the kinetics of vascular endothelial growth factor gene expression in ischemic myocardium. Circulation 110:685–691
Aboutaleb N, Shamsaei N, Khaksari M et al (2015) Pre-ischemic exercise reduces apoptosis in hippocampal CA3 cells after cerebral ischemia by modulation of the Bax/Bcl-2 proteins ratio and prevention of caspase-3 activation. J Physiol Sci 65:435–443
Jr SJ, Rodrigues FF, Izaki M et al (2014) Low-carbohydrate diet versus euglycemic hyperinsulinemic clamp for the assessment of myocardial viability with 18F-fluorodeoxyglucose-PET: a pilot study. Int J Cardiovasc Imaging 30(2):415–423
Reshef A, Shirvan A, Akselrodballin A et al (2010) Small-molecule biomarkers for clinical PET imaging of apoptosis. J Nucl Med 51:837–840
Niu G, Hen X (2010) Apoptosis imaging: beyond annexin V. J Nucl Med 51:1659–1662
Sun T, Tang G, Hua T et al (2015) Positron emission tomography imaging of cardiomyocyte apoptosis with a novel molecule probe [18F]FP-DPAZn2. Oncotarget 6:30579–30591
Han JH, Lim SY, Lee MS et al (2015) Sodium [18F]fluoride PET/CT in myocardial infarction. Mol Imaging Biol 17:214–221
Bialik S, Geenen DL, Sasson IE et al (1997) Myocyte apoptosis during acute myocardial infarction in the mouse localizes to hypoxic regions but occurs independently of p53. J Clin Invest 100:1363–1372
Acknowledgements
This work was supported in part by the Science and Technology Foundation of Guangdong Province (Nos. 2014A020210008 and 2016B090920087), the Science and Technology Planning Project Foundation of Guangzhou (Nos. 201604020169 and 201510010145), the National Natural Science Foundation of China (Nos. 81571704, 81671719 and 81770505), the Natural Science Foundation of Guangdong Province (No. 2015A030313067), and the Research Project of Shanghai Municipal Health and Family Planning Commission (No. 201740060).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ma, H., Liu, S., Xiong, Y. et al. PET imaging of cardiomyocyte apoptosis in a rat myocardial infarction model. Apoptosis 23, 396–407 (2018). https://doi.org/10.1007/s10495-018-1463-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-018-1463-x