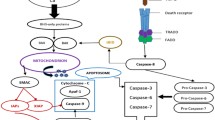
Abstract
Cancer is a primary cause of human fatality and conventional cancer therapies, e.g., chemotherapy, are often associated with adverse side-effects, tumor drug-resistance, and recurrence. Molecularly targeted therapy, composed of small-molecule inhibitors and immunotherapy (e.g., monoclonal antibody and cancer vaccines), is a less harmful alternative being more effective against cancer cells whilst preserving healthy tissues. Drug-resistance, however, caused by negative regulation of cell death signaling pathways, is still a challenge. Circumvention of negative regulators of cell death pathways or development of predictive and response biomarkers is, therefore, quintessential. This review critically discusses the current state of knowledge on targeting negative regulators of cell death signaling pathways including apoptosis, ferroptosis, necroptosis, autophagy, and anoikis and evaluates the recent advances in clinical and preclinical research on biomarkers of negative regulators. It aims to provide a comprehensive platform for designing efficacious polytherapies including novel agents for restoring cell death signaling pathways or targeting alternative resistance pathways to improve the chances for antitumor responses. Overall, it is concluded that nonapoptotic cell death pathways are a potential research arena for drug discovery, development of novel biomarkers and targeted therapies.


Similar content being viewed by others
References
Biemar F, Foti M (2013) Global progress against cancer-challenges and opportunities. Cancer Biol Med 10:183–186. https://doi.org/10.7497/j.issn.2095-3941.2013.04.001
Huang M, Shen A, Ding J, Geng M (2014) Molecularly targeted cancer therapy: some lessons from the past decade. Trends Pharmacol Sci 35:41–50. https://doi.org/10.1016/j.tips.2013.11.004
Lambert G, Estevez-Salmeron L, Oh S et al (2011) An analogy between the evolution of drug resistance in bacterial communities and malignant tissues. Nat Rev Cancer 11:375–382. https://doi.org/10.1038/nrc3039
Housman G, Byler S, Heerboth S et al (2014) Drug resistance in cancer: an overview. Cancers 6:1769–1792. https://doi.org/10.3390/cancers6031769
Hata AN, Engelman JA, Faber AC (2015) The BCL2 family: key mediators of the apoptotic response to targeted anticancer therapeutics. Cancer Discov 5:475–487. https://doi.org/10.1158/2159-8290.CD-15-0011
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. https://doi.org/10.1016/j.cell.2011.02.013
Garraway LA, Janne PA (2012) Circumventing cancer drug resistance in the era of personalized medicine. Cancer Discov 2:214–226. https://doi.org/10.1158/2159-8290.CD-12-0012
Wong RS (2011) Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res 30:87. https://doi.org/10.1186/1756-9966-30-87
Kasibhatla S, Tseng B (2003) Why target apoptosis in cancer treatment? Mol Cancer Ther 2:573–580
Xie Y, Hou W, Song X et al (2016) Ferroptosis: process and function. Cell Death Differ 23:369–379. https://doi.org/10.1038/cdd.2015.158
Razaghi A, Owens L, Heimann K (2016) Review of the recombinant human interferon gamma as an immunotherapeutic: impacts of production platforms and glycosylation. J Biotechnol 240:48–60. https://doi.org/10.1016/j.jbiotec.2016.10.022
Lee HJ, Kim JY, Park JE, Yoon YD, Tsang BK, Kim JM (2016) Induction of Fas-mediated apoptosis by interferon-gamma is dependent on granulosa cell differentiation and follicular maturation in the rat ovary. Dev Reprod 20:315–329. https://doi.org/10.12717/DR.2016.20.4.315
Razaghi A, Villacres C, Jung V et al (2017) Improved therapeutic efficacy of mammalian expressed-recombinant interferon gamma against ovarian cancer cells. Exp Cell Res 359:20–29. https://doi.org/10.1016/j.yexcr.2017.08.014
Guicciardi ME, Gores GJ (2009) Life and death by death receptors. FASEB J 23:1625–1637. https://doi.org/10.1096/fj.08-111005
Iyer S, Bell F, Westphal D et al (2015) Bak apoptotic pores involve a flexible C-terminal region and juxtaposition of the C-terminal transmembrane domains. Cell Death Differ 22:1665–1675. https://doi.org/10.1038/cdd.2015.15
Pobezinskaya YL, Liu Z (2012) The role of TRADD in death receptor signaling. Cell Cycle 11:871–876. https://doi.org/10.4161/cc.11.5.19300
Allen JE, Kline CL, Prabhu VV et al (2016) Discovery and clinical introduction of first-in-class imipridone ONC201. Oncotarget 7:74380–74392. https://doi.org/10.18632/oncotarget.11814
von Karstedt S, Conti A, Nobis M et al (2015) Cancer cell-autonomous TRAIL-R signaling promotes KRAS-driven cancer progression, invasion, and metastasis. Cancer Cell 27:561–573. https://doi.org/10.1016/j.ccell.2015.02.014
Chen L, Park SM, Tumanov AV et al (2010) CD95 promotes tumour growth. Nature 465:492–496. https://doi.org/10.1038/nature09075
Hartwig T, Montinaro A, von Karstedt S et al (2017) The TRAIL-Induced cancer secretome promotes a tumor-supportive immune microenvironment via CCR2. Mol Cell 65:730–742 e735. https://doi.org/10.1016/j.molcel.2017.01.021
Shirley S, Micheau O (2013) Targeting c-FLIP in cancer. Cancer Lett 332:141–150. https://doi.org/10.1016/j.canlet.2010.10.009
Fox JL, MacFarlane M (2016) Targeting cell death signalling in cancer: minimising ‘Collateral damage’. Br J Cancer 115:5–11. https://doi.org/10.1038/bjc.2016.111
Lemke J, von Karstedt S, Zinngrebe J, Walczak H (2014) Getting TRAIL back on track for cancer therapy. Cell Death Differ 21:1350–1364. https://doi.org/10.1038/cdd.2014.81
Lee EW, Seo J, Jeong M, Lee S, Song J (2012) The roles of FADD in extrinsic apoptosis and necroptosis. BMB Rep 45:496–508. https://doi.org/10.5483/BMBRep.2012.45.9.186
Zhang Y, Zhu J, Tang Y et al (2011) X-linked inhibitor of apoptosis positive nuclear labeling: a new independent prognostic biomarker of breast invasive ductal carcinoma. Diagn Pathol 6:49. https://doi.org/10.1186/1746-1596-6-49
Lafont E, Kantari-Mimoun C, Draber P et al (2017) The linear ubiquitin chain assembly complex regulates TRAIL-induced gene activation and cell death. EMBO J 36:1147–1166. https://doi.org/10.15252/embj.201695699
Probst BL, Liu L, Ramesh V et al (2010) Smac mimetics increase cancer cell response to chemotherapeutics in a TNF-alpha-dependent manner. Cell Death Differ 17:1645–1654. https://doi.org/10.1038/cdd.2010.44
Vamos M, Welsh K, Finlay D et al (2013) Expedient synthesis of highly potent antagonists of inhibitor of apoptosis proteins (IAPs) with unique selectivity for ML-IAP. ACS Chem Biol 8:725–732. https://doi.org/10.1021/cb3005512
Fenstermaker RA (2014) Survivin as a cancer vaccine target. J Vaccines Vaccin. https://doi.org/10.4172/2157-7560.1000230
Ausserlechner MJ, Hagenbuchner J (2016) Mitochondrial survivin—an Achilles’ heel in cancer chemoresistance. Mol Cell Oncol 3:e1076589. https://doi.org/10.1080/23723556.2015.1076589
Pan R, Hogdal LJ, Benito JM et al (2014) Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov 4:362–375. https://doi.org/10.1158/2159-8290.CD-13-0609
Cang S, Iragavarapu C, Savooji J, Song Y, Liu D (2015) ABT-199 (venetoclax) and BCL-2 inhibitors in clinical development. J Hematol Oncol 8:129. https://doi.org/10.1186/s13045-015-0224-3
Rudin CM, Hann CL, Garon EB et al (2012) Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin Cancer Res 18:3163–3169. https://doi.org/10.1158/1078-0432.CCR-11-3090
Del Poeta G, Postorino M, Pupo L et al (2016) Venetoclax: Bcl-2 inhibition for the treatment of chronic lymphocytic leukemia. Drugs Today 52:249–260. https://doi.org/10.1358/dot.2016.52.4.2470954
Inoue-Yamauchi A, Jeng PS, Kim K et al (2017) Targeting the differential addiction to anti-apoptotic BCL-2 family for cancer therapy. Nat Commun 8:16078. https://doi.org/10.1038/ncomms16078
Or CR, Chang Y, Lin WC et al. (2016) Obatoclax, a pan-BCL-2 inhibitor, targets cyclin D1 for degradation to induce antiproliferation in human colorectal carcinoma cells. Int J Mol Sci 18. https://doi.org/10.3390/ijms18010044
Oki Y, Copeland A, Hagemeister F et al (2012) Experience with obatoclax mesylate (GX15–070), a small molecule pan-Bcl-2 family antagonist in patients with relapsed or refractory classical Hodgkin lymphoma. Blood 119:2171–2172. https://doi.org/10.1182/blood-2011-11-391037
Safa AR (2016) Resistance to cell death and its modulation in cancer stem cells. Crit Rev Oncog 21:203–219. https://doi.org/10.1615/CritRevOncog.2016016976
Burton TR, Eisenstat DD, Gibson SB (2009) BNIP3 (Bcl-2 19 kDa interacting protein) acts as transcriptional repressor of apoptosis-inducing factor expression preventing cell death in human malignant gliomas. J Neurosci 29:4189–4199. https://doi.org/10.1523/JNEUROSCI.5747-08.2009
Li F, Zhang J, Arfuso F et al (2015) NF-κB in cancer therapy. Arch Toxicol 89:711–731. https://doi.org/10.1007/s00204-015-1470-4
Godwin P, Baird AM, Heavey S, Barr MP, O’Byrne KJ, Gately K (2013) Targeting nuclear factor-kappa B to overcome resistance to chemotherapy. Front Oncol 3:120. https://doi.org/10.3389/fonc.2013.00120
Douer D (2003) Arsenic trioxide (Trisenox(R)) therapy for acute promyelocytic leukemia in the setting of hematopoietic stem cell transplantation. Oncologist 8:132–140. https://doi.org/10.1634/theoncologist.8-2-132
Shono Y, Tuckett AZ, Liou HC et al (2016) Characterization of a c-Rel inhibitor that mediates anticancer properties in hematologic malignancies by blocking NF-kB-controlled oxidative stress responses. Cancer Res 76:377–389. https://doi.org/10.1158/0008-5472.CAN-14-2814
Fabre C, Mimura N, Bobb K et al (2012) Dual inhibition of canonical and noncanonical NF-kB pathways demonstrates significant antitumor activities in multiple myeloma. Clin Cancer Res 18:4669–4681. https://doi.org/10.1158/1078-0432.CCR-12-0779
Yamamoto M, Horie R, Takeiri M, Kozawa I, Umezawa K (2008) Inactivation of NF-kB components by covalent binding of (-)-dehydroxymethylepoxyquinomicin to specific cysteine residues. J Med Chem 51:5780–5788. https://doi.org/10.1021/jm8006245
Vaisitti T, Gaudino F, Ouk S et al (2017) Targeting metabolism and survival in chronic lymphocytic leukemia and Richter syndrome cells by a novel NF-kappaB inhibitor. Haematologica 102:1878–1889. https://doi.org/10.3324/haematol.2017.173419
Blakely CM, Pazarentzos E, Olivas V et al (2015) NF-kappaB-activating complex engaged in response to EGFR oncogene inhibition drives tumor cell survival and residual disease in lung cancer. Cell Rep 11:98–110. https://doi.org/10.1016/j.celrep.2015.03.012
Hughes MA, Powley IR, Jukes-Jones R et al (2016) Co-operative and hierarchical binding of c-FLIP and caspase-8: a unified model defines how c-FLIP isoforms differentially control cell fate. Mol Cell 61:834–849. https://doi.org/10.1016/j.molcel.2016.02.023
Longley DB, Wilson TR, McEwan M et al (2006) c-FLIP inhibits chemotherapy-induced colorectal cancer cell death. Oncogene 25:838–848. https://doi.org/10.1038/sj.onc.1209122
Schinske KA, Nyati S, Khan AP et al (2011) A novel kinase inhibitor of FADD phosphorylation chemosensitizes through the inhibition of NF-kappaB. Mol Cancer Ther 10:1807–1817. https://doi.org/10.1158/1535-7163.MCT-11-0362
Ramos-Miguel A, Garcia-Fuster MJ, Callado LF, La Harpe R, Meana JJ, Garcia-Sevilla JA (2009) Phosphorylation of FADD (Fas-associated death domain protein) at serine 194 is increased in the prefrontal cortex of opiate abusers: relation to mitogen activated protein kinase, phosphoprotein enriched in astrocytes of 15 kDa, and Akt signaling pathways involved in neuroplasticity. Neuroscience 161:23–38. https://doi.org/10.1016/j.neuroscience.2009.03.028
Nitulescu GM, Margina D, Juzenas P et al (2016) Akt inhibitors in cancer treatment: the long journey from drug discovery to clinical use (review). Int J Oncol 48:869–885. https://doi.org/10.3892/ijo.2015.3306
Yap TA, Yan L, Patnaik A et al (2011) First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol 29:4688–4695. https://doi.org/10.1200/JCO.2011.35.5263
Altomare DA, Testa JR (2005) Perturbations of the AKT signaling pathway in human cancer. Oncogene 24:7455–7464. https://doi.org/10.1038/sj.onc.1209085
Oki Y, Fanale M, Romaguera J et al (2015) Phase II study of an AKT inhibitor MK2206 in patients with relapsed or refractory lymphoma. Br J Haematol 171:463–470. https://doi.org/10.1111/bjh.13603
Ghobrial IM, Roccaro A, Hong F et al (2010) Clinical and translational studies of a phase II trial of the novel oral Akt inhibitor perifosine in relapsed or relapsed/refractory Waldenstrom’s macroglobulinemia. Clin Cancer Res 16:1033–1041. https://doi.org/10.1158/1078-0432.CCR-09-1837
Pal SK, Reckamp K, Yu H, Figlin RA (2010) Akt inhibitors in clinical development for the treatment of cancer. Expert Opin Investig Drugs 19:1355–1366. https://doi.org/10.1517/13543784.2010.520701
Porta C, Paglino C, Mosca A (2014) Targeting PI3K/Akt/mTOR signaling in cancer. Front Oncol 4:64. https://doi.org/10.3389/fonc.2014.00064
Henson E, Chen Y, Gibson S (2017) EGFR family members’ regulation of autophagy is at a crossroads of cell survival and death in cancer. Cancers. https://doi.org/10.3390/cancers9040027
Wei Y, Zou Z, Becker N et al (2013) EGFR-mediated Beclin 1 phosphorylation in autophagy suppression, tumor progression, and tumor chemoresistance. Cell 154:1269–1284. https://doi.org/10.1016/j.cell.2013.08.015
Ying WZ, Zhang HG, Sanders PW (2007) EGF receptor activity modulates apoptosis induced by inhibition of the proteasome of vascular smooth muscle cells. J Am Soc Nephrol 18:131–142. https://doi.org/10.1681/ASN.2006040333
Patrick A, Blevins M, Krueger A et al (2014) Abstract B102: targeting the SIX1/EYA transcriptional complex as a potential anti-cancer therapy. Mol Cancer Ther 12:B102-B102. https://doi.org/10.1158/1535-7163.targ-13-b102
Wang H, Li X, Liu H et al (2016) Six1 induces protein synthesis signaling expression in duck myoblasts mainly via up-regulation of mTOR. Genet Mol Biol 39:151–161. https://doi.org/10.1590/1678-4685-GMB-2015-0075
Armat M, Bakhshaiesh TO, Sabzichi M et al (2016) The role of Six1 signaling in paclitaxel-dependent apoptosis in MCF-7 cell line. Bosn J Basic Med Sci 16:28–34. https://doi.org/10.17305/bjbms.2016.674
Blevins MA, Towers CG, Patrick AN, Zhao R, Ford HL (2015) The SIX1-EYA transcriptional complex as a therapeutic target in cancer. Expert Opin Ther Targets 19:213–225. https://doi.org/10.1517/14728222.2014.978860
Zeng J, Liu D, Qiu Z et al (2014) GSK3beta overexpression indicates poor prognosis and its inhibition reduces cell proliferation and survival of non-small cell lung cancer cells. PLoS ONE 9:e91231. https://doi.org/10.1371/journal.pone.0091231
Dixon SJ, Lemberg KM, Lamprecht MR et al (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149:1060–1072. https://doi.org/10.1016/j.cell.2012.03.042
Jego G, Hazoume A, Seigneuric R, Garrido C (2013) Targeting heat shock proteins in cancer. Cancer Lett 332:275–285. https://doi.org/10.1016/j.canlet.2010.10.014
Chatterjee S, Burns TF. (2017) Targeting heat shock proteins in cancer: a promising therapeutic approach. Int J Mol Sci 18. https://doi.org/10.3390/ijms18091978
Sakamoto H, Mashima T, Yamamoto K, Tsuruo T (2002) Modulation of heat-shock protein 27 (Hsp27) anti-apoptotic activity by methylglyoxal modification. J Biol Chem 277:45770–45775. https://doi.org/10.1074/jbc.M207485200
Oba M, Yano S, Shuto T, Suico M, Eguma A, Kai H (2008) IFN-γ down-regulates Hsp27 and enhances hyperthermia-induced tumor cell death in vitro and tumor suppression in vivo. Int J Oncol. https://doi.org/10.3892/ijo.32.6.1317
Paoli P, Giannoni E, Chiarugi P (2013) Anoikis molecular pathways and its role in cancer progression. Biochim Biophys Acta 1833:3481–3498. https://doi.org/10.1016/j.bbamcr.2013.06.026
Su Z, Yang Z, Xie L, DeWitt JP, Chen Y (2016) Cancer therapy in the necroptosis era. Cell Death Differ 23:748–756. https://doi.org/10.1038/cdd.2016.8
Lalaoui N, Brumatti G (2017) Relevance of necroptosis in cancer. Immunol Cell Biol 95:137–145. https://doi.org/10.1038/icb.2016.120
Seo J, Lee E-W, Song J (2016) New role of E3 ubiquitin ligase in the regulation of necroptosis. BMB Reports 49:247–248. https://doi.org/10.5483/BMBRep.2016.49.5.067
Seo J, Lee E-W, Sung H et al (2016) CHIP controls necroptosis through ubiquitylation- and lysosome-dependent degradation of RIPK3. Nat Cell Biol 18:291–302. https://doi.org/10.1038/ncb3314
Osborn SL, Diehl G, Han SJ et al (2010) Fas-associated death domain (FADD) is a negative regulator of T-cell receptor-mediated necroptosis. Proc Natl Acad Sci USA 107:13034–13039. https://doi.org/10.1073/pnas.1005997107
Yang YP, Hu LF, Zheng HF et al (2013) Application and interpretation of current autophagy inhibitors and activators. Acta Pharmacol Sin 34:625–635. https://doi.org/10.1038/aps.2013.5
Polivka J Jr, Janku F (2014) Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol Ther 142:164–175. https://doi.org/10.1016/j.pharmthera.2013.12.004
Liang C (2010) Negative regulation of autophagy. Cell Death Differ 17:1807–1815. https://doi.org/10.1038/cdd.2010.115
Atkins MB, Yasothan U, Kirkpatrick P (2009) Everolimus. Nat Rev Drug Discov 8:535–536. https://doi.org/10.1038/nrd2924
Popovics P, Frigo DE, Schally AV, Rick FG (2015) Targeting the 5′-AMP-activated protein kinase and related metabolic pathways for the treatment of prostate cancer. Expert Opin Ther Targets 19:617–632. https://doi.org/10.1517/14728222.2015.1005603
Rehman G, Shehzad A, Khan AL, Hamayun M (2014) Role of AMP-activated protein kinase in cancer therapy. Arch Pharm 347:457–468. https://doi.org/10.1002/ardp.201300402
Faubert B, Boily G, Izreig S et al (2013) AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab 17:113–124. https://doi.org/10.1016/j.cmet.2012.12.001
Melosky B (2014) Review of EGFR TKIs in metastatic NSCLC, including ongoing trials. Front Oncol 4:244. https://doi.org/10.3389/fonc.2014.00244
Yang WS, SriRamaratnam R, Welsch ME et al (2014) Regulation of ferroptotic cancer cell death by GPX4. Cell 156:317–331. https://doi.org/10.1016/j.cell.2013.12.010
Dixon SJ, Stockwell BR (2014) The role of iron and reactive oxygen species in cell death. Nat Chem Biol 10:9–17. https://doi.org/10.1038/nchembio.1416
Ma S, Henson ES, Chen Y, Gibson SB (2016) Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells. Cell Death Dis 7:e2307. https://doi.org/10.1038/cddis.2016.208
Drakes ML, Stiff PJ (2014) Harnessing immunosurveillance: current developments and future directions in cancer immunotherapy. Immunotargets Ther 3:151–165. https://doi.org/10.2147/ITT.S37790
Weiner LM, Murray JC, Shuptrine CW (2012) Antibody-based immunotherapy of cancer. Cell 148:1081–1084. https://doi.org/10.1016/j.cell.2012.02.034
Guo C, Manjili MH, Subjeck JR, Sarkar D, Fisher PB, Wang XY (2013) Therapeutic cancer vaccines: past, present, and future. Adv Cancer Res 119:421–475. https://doi.org/10.1016/B978-0-12-407190-2.00007-1
Wilken JA, Webster KT, Maihle NJ (2010) Trastuzumab sensitizes ovarian cancer cells to EGFR-targeted therapeutics. J Ovarian Res 3:7. https://doi.org/10.1186/1757-2215-3-7
Sotelo MJ, Garcia-Paredes B, Aguado C, Sastre J, Diaz-Rubio E (2014) Role of cetuximab in first-line treatment of metastatic colorectal cancer. World J Gastroenterol 20:4208–4219. https://doi.org/10.3748/wjg.v20.i15.4208
Scott AM, Wolchok JD, Old LJ (2012) Antibody therapy of cancer. Nat Rev Cancer 12:278–287. https://doi.org/10.1038/nrc3236
Patel SB, Gill D, Garrido-Laguna I (2016) Profile of panitumumab as first-line treatment in patients with wild-type KRAS metastatic colorectal cancer. Onco Targets Ther 9:75–86. https://doi.org/10.2147/OTT.S68558
Garnock-Jones KP, Keating GM, Scott LJ (2010) Trastuzumab: a review of its use as adjuvant treatment in human epidermal growth factor receptor 2 (HER2)-positive early breast cancer. Drugs 70:215–239. https://doi.org/10.2165/11203700-000000000-00000
Rodriguez PC, Popa X, Martinez O et al (2016) A phase III clinical trial of the epidermal growth factor vaccine CIMAvax-EGF as switch maintenance therapy in advanced non-small cell lung cancer patients. Clin Cancer Res 22:3782–3790. https://doi.org/10.1158/1078-0432.CCR-15-0855
Saavedra D, Crombet T (2017) CIMAvax-EGF: a new therapeutic vaccine for advanced non-small cell lung cancer patients. Front Immunol 8:269. https://doi.org/10.3389/fimmu.2017.00269
Hirschowitz EA, Foody T, Hidalgo GE, Yannelli JR (2007) Immunization of NSCLC patients with antigen-pulsed immature autologous dendritic cells. Lung Cancer 57:365–372. https://doi.org/10.1016/j.lungcan.2007.04.002
Tanaka T, Kitamura H, Inoue R et al. (2013) Potential survival benefit of anti-apoptosis protein: survivin-derived peptide vaccine with and without interferon alpha therapy for patients with advanced or recurrent urothelial cancer—results from phase I clinical trials. Clin Dev Immunol 2013:262967. https://doi.org/10.1155/2013/262967
Fenstermaker RA, Ciesielski MJ, Qiu J et al (2016) Clinical study of a survivin long peptide vaccine (SurVaxM) in patients with recurrent malignant glioma. Cancer Immunol Immunother 65:1339–1352. https://doi.org/10.1007/s00262-016-1890-x
Li R, Qian J, Zhang W et al (2014) Human heat shock protein-specific cytotoxic T lymphocytes display potent antitumour immunity in multiple myeloma. Br J Haematol 166:690–701. https://doi.org/10.1111/bjh.12943
Bloch O, Crane CA, Fuks Y et al (2014) Heat-shock protein peptide complex-96 vaccination for recurrent glioblastoma: a phase II, single-arm trial. Neuro Oncol 16:274–279. https://doi.org/10.1093/neuonc/not203
Henry NL, Hayes DF (2012) Cancer biomarkers. Mol Oncol 6:140–146. https://doi.org/10.1016/j.molonc.2012.01.010
Ward TH, Cummings J, Dean E et al (2008) Biomarkers of apoptosis. Br J Cancer 99:841–846. https://doi.org/10.1038/sj.bjc.6604519
Krysan K, Dalwadi H, Sharma S, Pold M, Dubinett S (2004) Cyclooxygenase 2-dependent expression of survivin is critical for apoptosis resistance in non-small cell lung cancer. Cancer Res 64:6359–6362. https://doi.org/10.1158/0008-5472.CAN-04-1681
Olsen D, Jorgensen JT (2014) Companion diagnostics for targeted cancer drugs - clinical and regulatory aspects. Front Oncol 4:105. https://doi.org/10.3389/fonc.2014.00105
Punnoose EA, Leverson JD, Peale F et al (2016) Expression profile of BCL-2, BCL-XL, and MCL-1 predicts pharmacological response to the BCL-2 selective antagonist Venetoclax in multiple myeloma models. Mol Cancer Ther 15:1132–1144. https://doi.org/10.1158/1535-7163.MCT-15-0730
Khalil AA, Kabapy NF, Deraz SF, Smith C (2011) Heat shock proteins in oncology: diagnostic biomarkers or therapeutic targets? Biochim Biophys Acta 1816:89–104. https://doi.org/10.1016/j.bbcan.2011.05.001
Ciocca DR, Calderwood SK (2005) Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 10:86. https://doi.org/10.1379/CSC-99r.1
Del Gaizo Moore V, Letai A (2013) BH3 profiling—measuring integrated function of the mitochondrial apoptotic pathway to predict cell fate decisions. Cancer Lett 332:202–205. https://doi.org/10.1016/j.canlet.2011.12.021
Callagy GM, Pharoah PD, Pinder SE et al (2006) Bcl-2 is a prognostic marker in breast cancer independently of the Nottingham Prognostic Index. Clin Cancer Res 12:2468–2475. https://doi.org/10.1158/1078-0432.CCR-05-2719
Yoshino T, Shiina H, Urakami S et al (2006) Bcl-2 expression as a predictive marker of hormone-refractory prostate cancer treated with taxane-based chemotherapy. Clin Cancer Res 12:6116–6124. https://doi.org/10.1158/1078-0432.CCR-06-0147
Jaiswal PK, Goel A, Mittal RD (2015) Survivin: a molecular biomarker in cancer. Indian J Med Res 141:389–397. https://doi.org/10.4103/0971-5916.159250
Seligson DB, Hongo F, Huerta-Yepez S et al (2007) Expression of X-linked inhibitor of apoptosis protein is a strong predictor of human prostate cancer recurrence. Clin Cancer Res 13:6056–6063. https://doi.org/10.1158/1078-0432.CCR-07-0960
Dutton A, Young LS, Murray PG (2006) The role of cellular FLICE inhibitory protein (c-FLIP) in the pathogenesis and treatment of cancer. Expert Opin Ther Targets 10:27–35. https://doi.org/10.1517/14728222.10.1.27
Korkolopoulou P, Saetta AA, Levidou G et al (2007) c-FLIP expression in colorectal carcinomas: association with Fas/FasL expression and prognostic implications. Histopathology 51:150–156. https://doi.org/10.1111/j.1365-2559.2007.02723.x
Schrijvers ML, Pattje WJ, Slagter-Menkema L et al (2012) FADD expression as a prognosticator in early-stage glottic squamous cell carcinoma of the larynx treated primarily with radiotherapy. Int J Radiat Oncol Biol Phys 83:1220–1226. https://doi.org/10.1016/j.ijrobp.2011.09.060
Kurozumi S, Yamaguchi Y, Hayashi S et al (2016) Prognostic value of the ubiquitin ligase carboxyl terminus of the Hsc70-interacting protein in postmenopausal breast cancer. Cancer Med 5:1873–1882. https://doi.org/10.1002/cam4.780
Liang ZL, Kim M, Huang SM, Lee HJ, Kim JM (2013) Expression of carboxyl terminus of Hsp70-interacting protein (CHIP) indicates poor prognosis in human gallbladder carcinoma. Oncol Lett 5:813–818. https://doi.org/10.3892/ol.2013.1138
Owonikoko TK, Khuri FR (2013) Targeting the PI3K/AKT/mTOR pathway: biomarkers of success and tribulation. Am Soc Clin Oncol Educ Book. https://doi.org/10.1200/EdBook_AM.2013.33.e395
Zeng J, Shi R, Cai CX et al (2015) Increased expression of Six1 correlates with progression and prognosis of prostate cancer. Cancer Cell Int 15:63. https://doi.org/10.1186/s12935-015-0215-z
Guerriero E, Capone F, Accardo M et al (2015) GPX4 and GPX7 over-expression in human hepatocellular carcinoma tissues. Eur J Histochem 59:2540. https://doi.org/10.4081/ejh.2015.2540
Larrea E, Sole C, Manterola L et al. (2016) New concepts in cancer biomarkers: circulating miRNAs in liquid biopsies. Int J Mol Sci 17. https://doi.org/10.3390/ijms17050627
Diaz LA Jr, Bardelli A (2014) Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol 32:579–586. https://doi.org/10.1200/JCO.2012.45.2011
Oellerich M, Schutz E, Beck J et al (2017) Using circulating cell-free DNA to monitor personalized cancer therapy. Crit Rev Clin Lab Sci 54:205–218. https://doi.org/10.1080/10408363.2017.1299683
Vincent MD, Kuruvilla MS, Leighl NB, Kamel-Reid S (2012) Biomarkers that currently affect clinical practice: EGFR, ALK, MET, KRAS. Curr Oncol 19:S33–S44. https://doi.org/10.3747/co.19.1149
Page K, Hava N, Ward B et al (2011) Detection of HER2 amplification in circulating free DNA in patients with breast cancer. Br J Cancer 104:1342–1348. https://doi.org/10.1038/bjc.2011.89
Morin I, Dixon NE, Schaeffer PM (2010) Ultrasensitive detection of antibodies using a new Tus-Ter-lock immunoPCR system. Mol Biosyst 6:1173–1175. https://doi.org/10.1039/c002163f
Chang L, Li J, Wang L (2016) Immuno-PCR: an ultrasensitive immunoassay for biomolecular detection. Anal Chim Acta 910:12–24. https://doi.org/10.1016/j.aca.2015.12.039
Li K, Wu D, Chen X et al (2014) Current and emerging biomarkers of cell death in human disease. Biomed Res Int 2014:690103. https://doi.org/10.1155/2014/690103
Weigum SE, Floriano PN, Redding SW et al (2010) Nano-bio-chip sensor platform for examination of oral exfoliative cytology. Cancer Prev Res 3:518–528. https://doi.org/10.1158/1940-6207.CAPR-09-0139
Morin I, Askin SP, Schaeffer PM (2011) IgG-detection devices for the Tus-Ter-lock immuno-PCR diagnostic platform. Analyst 136:4815–4821. https://doi.org/10.1039/c1an15731k
Dahdah DB, Morin I, Moreau MJ, Dixon NE, Schaeffer PM. (2009) Site-specific covalent attachment of DNA to proteins using a photoactivatable Tus-Ter complex. Chem Commun (Camb):3050–3052. https://doi.org/10.1039/b900905a
Spencer KR, Wang J, Silk AW, Ganesan S, Kaufman HL, Mehnert JM (2016) Biomarkers for Immunotherapy: current developments and challenges. Am Soc Clin Oncol Educ Book 35:e493–e503. https://doi.org/10.14694/EDBK_160766
Qiu H, Fang X, Luo Q, Ouyang G (2015) Cancer stem cells: a potential target for cancer therapy. Cell Mol Life Sci 72:3411–3424. https://doi.org/10.1007/s00018-015-1920-4
Signore M, Ricci-Vitiani L, De Maria R (2013) Targeting apoptosis pathways in cancer stem cells. Cancer Lett 332:374–382. https://doi.org/10.1016/j.canlet.2011.01.013
Giampazolias E, Zunino B, Dhayade S et al (2017) Mitochondrial permeabilization engages NF-kappaB-dependent anti-tumour activity under caspase deficiency. Nat Cell Biol 19:1116–1129. https://doi.org/10.1038/ncb3596
Hannes S, Abhari BA, Fulda S (2016) Smac mimetic triggers necroptosis in pancreatic carcinoma cells when caspase activation is blocked. Cancer Lett 380:31–38. https://doi.org/10.1016/j.canlet.2016.05.036
Lopez JS, Banerji U (2017) Combine and conquer: challenges for targeted therapy combinations in early phase trials. Nat Rev Clin Oncol 14:57–66. https://doi.org/10.1038/nrclinonc.2016.96
Ricci MS, Zong WX (2006) Chemotherapeutic approaches for targeting cell death pathways. Oncologist 11:342–357. https://doi.org/10.1634/theoncologist.11-4-342
Grazia G, Penna I, Perotti V, Anichini A, Tassi E (2014) Towards combinatorial targeted therapy in melanoma: from pre-clinical evidence to clinical application (review). Int J Oncol 45:929–949. https://doi.org/10.3892/ijo.2014.2491
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10495_2018_1440_MOESM1_ESM.mp4
Supplementary Video 1. Anti-apoptotic Bcl-xl protein negatively regulates Fas-mediated apoptosis. Subsequently, SMI deactivates Bcl-xl and unblocks the signal transduction pathway, restoring apoptosis. Bax, Bcl-2-associated X protein; BID, BH3 interacting-domain death agonist; Casp-8, caspase-8; CYT-C, cytochrome-c; FADD, Fas-associated protein with death domain; FasL, Fas ligand; RIP, receptor interacting protein; SMI, small-molecule inhibitor; tBID, truncated BID (MP4 7150 KB)
Rights and permissions
About this article
Cite this article
Razaghi, A., Heimann, K., Schaeffer, P.M. et al. Negative regulators of cell death pathways in cancer: perspective on biomarkers and targeted therapies. Apoptosis 23, 93–112 (2018). https://doi.org/10.1007/s10495-018-1440-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-018-1440-4