Abstract
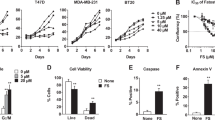
Fatty acid synthase (FASN) is a key enzyme in fat biosynthesis that is over-expressed in advanced breast cancer stages. Cisplatin (CDDP) is a platinum-based drug used in the treatment of certain types of this disease. Although it was shown that FASN inhibition induced apoptosis by enhancing the cytotoxicity of certain drugs in breast cancer, its role in regulating the chemosensitivity of different types of breast cancer cells to CDDP-induced apoptosis is not established yet. Therefore, two different breast cancer cell lines; triple negative breast cancer (TNBC; MDA-MB-231) and triple positive breast cancer (TPBC; BT-474) cells were used to examine such role. We show that TNBC cells had naturally less fat content than TPBC cells. Subsequently, the fat content increased in both cells when treated with Palmitate rather than Oleate, whereas both fatty acids produced apoptotic ultra-structural effects and attenuated FASN expression. However, Oleate increased FASN expression in TPBC cells. CDDP decreased FASN expression and increased apoptosis in TNBC cells. These effects were further enhanced by combining CDDP with fatty acids. We also illustrate that the inhibition of FASN by either siRNA or exogenous inhibitor decreased CDDP-induced apoptosis in TPBC cells suggesting its role as an apoptotic factor, while an opposite finding was observed in TNBC cells when siRNA and fatty acids were used, suggesting its role as a survival factor. To our knowledge, we are the first to demonstrate a dual role of FASN in CDDP-induced apoptosis in breast cancer cells and how it can modulate their chemosensitivity.





Similar content being viewed by others
References
WHO Cancer. http://www.who.int/cancer/en/. Accessed 22 April 2015
Zeng L, Biernacka K, Holly J, Jarrett C, Morrison A, Morgan A, Winters Z, Foulstone E, Shield J, Perks C (2010) Hyperglycaemia confers resistance to chemotherapy on breast cancer cells: the role of fatty acid synthase. Endocr Relat Cancer 17(2):539–551
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin CA 61(2):69–90
Kumar S, Burney I, Al-Ajmi A, Al-Moundhri M (2011) Changing trends of breast cancer survival in Sultanate of Oman. J Oncol 10:7
Cleator S, Heller W, Coombes R (2007) Triple-negative breast cancer: therapeutic options. Lancet Oncol 8:235–244
Sirohi B, Arnedos M, Popat S, Ashley S, Nerurkar A, Walsh G, Johnston S, Smith I (2008) Platinum-based chemotherapy in triple-negative breast cancer. Ann Oncol 19(11):1847–1852
Voduc K, Cheang M, Tyldesley S, Gelmon K, Nielsen T, Kennecke H (2010) Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol 28(10):1684–1691
Silver D, Richardson A, Eklund A, Wang Z, Szallasi Z, Li Q et al (2010) Efficacy of neoadjuvant cisplatin in triple-negative breast cancer. J Clin Oncol 28(7):1145–1153
Hardy S, Langelier Y, Prentki M (2000) Oleate activates phosphatidylinositol 3-kinase and promotes proliferation and reduces apoptosis of MDA-MB-231 breast cancer cells, whereas palmitate has opposite effects. Cancer Res 60:6353–6358
Hardy S, St-Onge G, Joly E, Langelier Y, Prentki M (2005) Oleate promotes the proliferation of breast cancer cells via the G protein-coupled receptor GPR40. J Biol Chem 280(14):13285–13291
Hardy S, El-Assaad W, Przybytkowski E, Joly E, Prentki M, Langelier Y (2003) Saturated fatty acid-induced apoptosis in MDA-MB-231 breast cancer cells: A ROLE FOR CARDIOLIPIN. J Biol Chem 278(34):31861–31870
Smith S (1994) The animal fatty acid synthase: one gene, one polypeptide, seven enzymes. FASEB J 8:1248–1259
Kuhajda FP (2006) Fatty acid synthase and cancer: new aplication of an old pathway. Cancer Res 66(12):5977–5980
Pizer E, Thupari J, Han W, Pinn M, Chrest F, Frehywot G, Townsend C, Kuhajda F (2000) Malonyl-coenzyme-A is a potential mediator of cytotoxicity induced by fatty-acid synthase inhibition in human breast cancer cells and xenografts. Cancer Res 60:213–218
Menendez J, Colomer R, Lupu R (2005) Why does tumor-associated fatty acid synthase (oncogenic antigen-519) ignore dietary fatty acids? Med Hypotheses 64(2):342–349
Mashima T, Seimiya H, Tsuruo T (2009) De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br J Cancer 100:1369–1372
Liu H, Liu Y, Zhang J (2008) A new mechanism of drug resistance in breast cancer cells: fatty acid synthase overexpression-mediated palmitate overproduction. Mol Cancer Ther 7(2):263–270
Migita T, Ruiz S, Fornari A, Fiorentino M, Priolo C, Zadra G et al (2009) Fatty acid synthase: a metabolic enzyme and candidate oncogene in prostate cancer. J Natl Cancer Inst 101(7):519–532
Yu D, Hung M (1999) Breast cancer chemosensitivity. Breast cancer. Springer, New York, pp 481–517
Zhao Y, Butler E, Tan M (2013) Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis 4(3):e532
Hooshmand S, Ghaderi A, Yusoff K, Thilakavathy K, Rosli R, Mojtahedi Z (2014) Asian Pac J Cancer Prev 15(7):3311–3317
Mellén M, de la Rosa E, Boya P (2008) The autophagic machinery is necessary for removal of cell corpses from the developing retinal neuroepithelium. Cell Death Differ 15(8):1279–1290
Al-Bahlani S, Fraser M, Wong A, Sayan B, Bergeron R, Melino G et al (2011) P73 regulates cisplatin-induced apoptosis in ovarian cancer cells via a calcium/calpain-dependent mechanism. Oncogene 30(41):4219–4230
Nieman K, Kenny H, Penicka C, Ladanyi A, BuellGutbrod R, Zillhardt M, Romero I, Carey M, Mills G, Hotamisligil G, Yamada S, Peter M, Gwin K, Lengyel E (2014) Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med 17(11): 1498–1503
Menendez J, Mehmi I, Atlas E, Colomer R, Lupu R (2004) Novel signaling molecules implicated in tumor-associated fatty acid synthase-dependent breast cancer cell proliferation and survival: role of exogenous dietary fatty acids, p53-p21WAF1/CIP1, ERK1/2 MAPK, p27KIP1, BRCA1, and NF-κB. Int J Oncol 24(3):591–608
Menendez J, Vellon L, Colomer R, Lupu R (2005) Pharmacological and small interference RNA-mediated inhibition of breast cancer-associated fatty acid synthase (oncogenic antigen-519) synergistically enhances Taxol (paclitaxel)-induced cytotoxicity. Int J Cancer 115(1):19–35
Pauzi A, Yeap S, Abu N, Lim K, Omar A, Aziz S et al (2016) Combination of cisplatin and bromelain exerts synergistic cytotoxic effects against breast cancer cell line MDA-MB-231 in vitro. Chin Med 11(1):46
Lehmann B, Bauer J, Chen X, Sanders M, Chakravarthy A, Shyr Y et al (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 121(7):2750–2767
Kao J, Salari K, Bocanegra M, Choi Y, Girard L, Gandhi J et al (2009) Molecular profiling of breast cancer cell lines defines relevant tumor models and provides a resource for cancer gene discovery. PLoS ONE 4(7):e6146
Zhang D (2005) Proteomic study reveals that proteins involved in metabolic and detoxification pathways are highly expressed in HER-2/neu-positive breast cancer. Mol Cell Proteom 4(11):1686–1696
Yoon S, Lee M, Park S, Moon J, Koh Y, Ahn Y et al (2007) Up-regulation of acetyl-CoA carboxylase and fatty acid synthase by human epidermal growth factor receptor 2 at the translational level in breast cancer cells. J Biol Chem 282(36):26122–26131
Yan C, Wei H, Minjuan Z, Yan X, Jingyue Y, Wenchao L et al (2014) The mTOR inhibitor rapamycin synergizes with a fatty acid synthase inhibitor to induce cytotoxicity in ER/HER2-positive breast cancer cells. PLoS ONE 9(5):e97697
Chamras H, Ardashian A, Heber D, Glaspy J (2002) Fatty acid modulation of MCF-7 human breast cancer cell proliferation, apoptosis and differentiation. J Nutr Biochem 107(30):13520–13525
Menendez J, Lupu R, Colomer R (2005) Targeting fatty acid synthase: potential for therapeutic intervention in her-2/neu-overexpressing breast cancer. Drug News Prospect 18(6):375–385
Solanas M, Hurtado A, Costa I, Moral R, Menendez J, Colomer R, Escrich E (2002) Effects of a high olive oil diet on the clinical behavior and histopathological feastures of rat DMBA-induced mammary tumors compared with a high corn oil diet. Int J Oncol 21(4):745–753
Kourtidis A, Srinivasaiah R, Carkner R, Brosnan M, Conklin D (2009) Peroxisome proliferator-activated receptor-γ protects ERBB2-positive breast cancer cells from palmitate toxicity. Breast Cancer Res 11(2):R16
Sheng X, Mittelman S (2014) The role of adipose tissue and obesity in causing treatment resistance of acute lymphoblastic leukemia. Front Pediatr 2:53
Ribeiro R, Monteiro C, Cunha V, Oliveira M, Freitas M, Fraga A et al (2012) Human periprostatic adipose tissue promotes prostate cancer aggressiveness in vitro. J Exp Clin Cancer Res 31(1):32
Pandey R P, Liu W, Xing F, Fukuda K, Watabe K (2012) Anti-cancer drugs targeting fatty acid synthase (FAS). Recent Pat Anti-Cancer Drug Discov 7(2):185–197
Li J, Gorospe M, Chrest F, Kumaravel T, Evans M, Han WF et al (2001) Pharmacological inhibition of fatty acid synthase activity produces both cytostatic and cytotoxic effects modulated by p53. Cancer Res 61:1493–1499
Vazquez-Martin A, Colomer R, Brunet J, Lupu R, Menendez J (2008) Overexpression of fatty acid synthase gene activates HER1/HER2 tyrosine kinase receptors in human breast epithelial cells. Cell Prolif 41(1):59–85
Alarmo E, Kallioniemi A (2010) Bone morphogenetic proteins in breast cancer: dual role in tumourigenesis?. Endocr Relat Cancer 17(2):R123–R139
Yamamoto T, Saatcioglu F, Matsuda T (2002) Cross-talk between bone morphogenic proteins and estrogen receptor signaling. Endocrinology 143(7):2635–2642
Vasiljeva O, Turk B (2008) Dual contrasting roles of cysteine cathepsins in cancer progression: apoptosis versus tumour invasion. Biochimie 90(2):380–386
López-Lázaro M (2007) Dual role of hydrogen peroxide in cancer: possible relevance to cancer chemoprevention and therapy. Cancer Lett 252(1):1–8
Sanchezcapelo A (2005) Dual role for TGF-β1 in apoptosis. Cytokine Growth Growth Rev 16(1):15–34
Acknowledgements
We thank Dr. Ikhlas Ahmed for her guidance and advices in this study. This research was supported by the department of Allied Health Sciences, College of Medicine and Health Sciences at Sultan Qaboos University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Al-Bahlani, S., Al-Lawati, H., Al-Adawi, M. et al. Fatty acid synthase regulates the chemosensitivity of breast cancer cells to cisplatin-induced apoptosis. Apoptosis 22, 865–876 (2017). https://doi.org/10.1007/s10495-017-1366-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-017-1366-2