Abstract
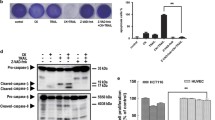
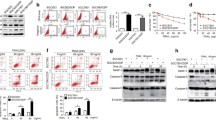
Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) is a potent cancer cell-specific apoptosis-inducing cytokine with little toxicity to most normal cells. Here, we report that gefitinib and TRAIL in combination produce a potent synergistic effect on TRAIL-sensitive human colon cancer HCT116 cells and an additive effect on TRAIL-resistant HT-29 cells. Interestingly, gefitinib increases the expression of cell surface receptors DR4 and DR5, possibly explaining the synergistic effect. Knockdown of DR4 and DR5 by siRNA significantly decreases gefitinib- and TRAIL-mediated cell apoptosis, supporting this idea. Because the inhibition of gefitinib-induced autophagy by 3-MA significantly decreases DR4 and DR5 upregulation, as well as reduces gefitinib- and TRAIL-induced apoptosis, we conclude that death receptor upregulation is autophagy mediated. Furthermore, our results indicate that death receptor expression may also be regulated by JNK activation, because pre-treatment of cells with JNK inhibitor SP600125 significantly decreases gefitinib-induced death receptor upregulation. Interestingly, SP600125 also inhibits the expression CHOP, yet CHOP has no impact on death receptor expressions. We also find here that phosphorylation of Akt and ERK might also be required for TRAIL sensitization. In summary, our results indicate that gefitinib effectively enhances TRAIL-induced apoptosis, likely via autophagy and JNK- mediated death receptor expression and phosphorylation of Akt and ERK.






Similar content being viewed by others
References
Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA et al (1995) Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 3(6):673–682
Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C, Smolak P, Goodwin RG, Rauch CT, Schuh JC, Lynch DH (1999) Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med 5(2):157–163. doi:10.1038/5517
Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH (1999) Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest 104(2):155–162. doi:10.1172/JCI6926
Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM (1997) An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science 277(5327):815–818
Chaudhary PM, Eby M, Jasmin A, Bookwalter A, Murray J, Hood L (1997) Death receptor 5, a new member of the TNFR family, and DR4 induce FADD-dependent apoptosis and activate the NF-kappaB pathway. Immunity 7(6):821–830
Pan G, O’Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, Dixit VM (1997) The receptor for the cytotoxic ligand TRAIL. Science 276(5309):111–113
Kimberley FC, Screaton GR (2004) Following a TRAIL: update on a ligand and its five receptors. Cell Res 14(5):359–372. doi:10.1038/sj.cr.7290236
Plummer R, Attard G, Pacey S, Li L, Razak A, Perrett R, Barrett M, Judson I, Kaye S, Fox NL, Halpern W, Corey A, Calvert H, de Bono J (2007) Phase 1 and pharmacokinetic study of lexatumumab in patients with advanced cancers. Clin Cancer Res 13(20):6187–6194. doi:10.1158/1078-0432.CCR-07-0950
Hotte SJ, Hirte HW, Chen EX, Siu LL, Le LH, Corey A, Iacobucci A, MacLean M, Lo L, Fox NL, Oza AM (2008) A phase 1 study of mapatumumab (fully human monoclonal antibody to TRAIL-R1) in patients with advanced solid malignancies. Clin Cancer Res 14(11):3450–3455. doi:10.1158/1078-0432.CCR-07-1416
Camidge DR, Herbst RS, Gordon MS, Eckhardt SG, Kurzrock R, Durbin B, Ing J, Tohnya TM, Sager J, Ashkenazi A, Bray G, Mendelson D (2010) A phase I safety and pharmacokinetic study of the death receptor 5 agonistic antibody PRO95780 in patients with advanced malignancies. Clin Cancer Res 16(4):1256–1263. doi:10.1158/1078-0432.CCR-09-1267
Cheng H, Hong B, Zhou L, Allen JE, Tai G, Humphreys R, Dicker DT, Liu YY, El-Deiry WS (2012) Mitomycin C potentiates TRAIL-induced apoptosis through p53-independent upregulation of death receptors: evidence for the role of c-Jun N-terminal kinase activation. Cell Cycle 11(17):3312–3323. doi:10.4161/cc.21670
Dolloff NG, Mayes PA, Hart LS, Dicker DT, Humphreys R, El-Deiry WS (2011) Off-target lapatinib activity sensitizes colon cancer cells through TRAIL death receptor up-regulation. Science Transl Med 3(86):86ra50. doi:10.1126/scitranslmed.3001384
Baselga J, Averbuch SD (2000) ZD1839 (‘Iressa’) as an anticancer agent. Drugs 60(Suppl 1):33–40 discussion 41–32
Baselga J, Rischin D, Ranson M, Calvert H, Raymond E, Kieback DG, Kaye SB, Gianni L, Harris A, Bjork T, Averbuch SD, Feyereislova A, Swaisland H, Rojo F, Albanell J (2002) Phase I safety, pharmacokinetic, and pharmacodynamic trial of ZD1839, a selective oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with five selected solid tumor types. J Clin Oncol 20(21):4292–4302
Wakeling AE, Guy SP, Woodburn JR, Ashton SE, Curry BJ, Barker AJ, Gibson KH (2002) ZD1839 (Iressa): an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res 62(20):5749–5754
Lorusso PM (2003) Phase I studies of ZD1839 in patients with common solid tumors. Semin Oncol 30 (Suppl 1):21–29. doi:10.1053/sonc.2003.50029
Schlessinger J (2000) Cell signaling by receptor tyrosine kinases. Cell 103(2):211–225
Sibilia M, Fleischmann A, Behrens A, Stingl L, Carroll J, Watt FM, Schlessinger J, Wagner EF (2000) The EGF receptor provides an essential survival signal for SOS-dependent skin tumor development. Cell 102(2):211–220
Gschwind A, Fischer OM, Ullrich A (2004) The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat Rev Cancer 4(5):361–370. doi:10.1038/nrc1360
Magne N, Fischel JL, Tiffon C, Formento P, Dubreuil A, Renee N, Formento JL, Francoual M, Ciccolini J, Etienne MC, Milano G (2003) Molecular mechanisms underlying the interaction between ZD1839 (‘Iressa’) and cisplatin/5-fluorouracil. Br J Cancer 89(3):585–592. doi:10.1038/sj.bjc.6601131
Tortora G, Caputo R, Damiano V, Melisi D, Bianco R, Fontanini G, Veneziani BM, De Placido S, Bianco AR, Ciardiello F (2003) Combination of a selective cyclooxygenase-2 inhibitor with epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 and protein kinase A antisense causes cooperative antitumor and antiangiogenic effect. Clin Cancer Res 9(4):1566–1572
Xu F, Tian Y, Huang Y, Zhang LL, Guo ZZ, Huang JJ, Lin TY (2011) EGFR inhibitors sensitize non-small cell lung cancer cells to TRAIL-induced apoptosis. Chin J Cancer 30(10):701–711. doi:10.5732/cjc.011.10107
Shrader M, Pino MS, Lashinger L, Bar-Eli M, Adam L, Dinney CP, McConkey DJ (2007) Gefitinib reverses TRAIL resistance in human bladder cancer cell lines via inhibition of AKT-mediated X-linked inhibitor of apoptosis protein expression. Cancer Res 67(4):1430–1435. doi:10.1158/0008-5472.CAN-06-1224
Teraishi F, Kagawa S, Watanabe T, Tango Y, Kawashima T, Umeoka T, Nisizaki M, Tanaka N, Fujiwara T (2005) ZD1839 (Gefitinib, ‘Iressa’), an epidermal growth factor receptor-tyrosine kinase inhibitor, enhances the anti-cancer effects of TRAIL in human esophageal squamous cell carcinoma. FEBS Lett 579 (19):4069–4075. doi:10.1016/j.febslet.2005.06.031
Yerbes R, Lopez-Rivas A, Reginato MJ, Palacios C (2012) Control of FLIP(L) expression and TRAIL resistance by the extracellular signal-regulated kinase1/2 pathway in breast epithelial cells. Cell Death Differ 19(12):1908–1916. doi:10.1038/cdd.2012.78
Yan D, Ge Y, Deng H, Chen W, An G (2015) Gefitinib upregulates death receptor 5 expression to mediate rmhTRAIL-induced apoptosis in Gefitinib-sensitive NSCLC cell line. Onco Targets Ther 8:1603–1610. doi:10.2147/OTT.S73731
Kim SH, Kim K, Kwagh JG, Dicker DT, Herlyn M, Rustgi AK, Chen Y, El-Deiry WS (2004) Death induction by recombinant native TRAIL and its prevention by a caspase 9 inhibitor in primary human esophageal epithelial cells. J Biol Chem 279(38):40044–40052. doi:10.1074/jbc.M404541200
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Han W, Pan H, Chen Y, Sun J, Wang Y, Li J, Ge W, Feng L, Lin X, Wang X, Jin H (2011) EGFR tyrosine kinase inhibitors activate autophagy as a cytoprotective response in human lung cancer cells. PloS One 6(6):e18691. doi:10.1371/journal.pone.0018691
Dragowska WH, Weppler SA, Wang JC, Wong LY, Kapanen AI, Rawji JS, Warburton C, Qadir MA, Donohue E, Roberge M, Gorski SM, Gelmon KA, Bally MB (2013) Induction of autophagy is an early response to gefitinib and a potential therapeutic target in breast cancer. PloS One 8(10):e76503. doi:10.1371/journal.pone.0076503
Han J, Hou W, Goldstein LA, Lu CS, Stolz DB, Yin XM, Rabinowich H (2008) Involvement of protective autophagy in TRAIL resistance of apoptosis-defective tumor cells. J Biol Chem 283(28):19665–19677. doi:10.1074/jbc.M710169200
Hou W, Han J, Lu C, Goldstein LA, Rabinowich H (2008) Enhancement of tumor-TRAIL susceptibility by modulation of autophagy. Autophagy 4(7):940–943
Yuan BZ, Chapman J, Ding M, Wang J, Jiang B, Rojanasakul Y, Reynolds SH (2013) TRAIL and proteasome inhibitors combination induces a robust apoptosis in human malignant pleural mesothelioma cells through Mcl-1 and Akt protein cleavages. BMC Cancer 13(1):140. doi:10.1186/1471-2407-13-140
Di X, Zhang G, Zhang Y, Takeda K, Rosado LA, Zhang B (2013) Accumulation of autophagosomes in breast cancer cells induces TRAIL resistance through downregulation of surface expression of death receptors 4 and 5. Oncotarget 4(9):1349–1364
Thamkachy R, Kumar R, Rajasekharan KN, Sengupta S (2016) ERK mediated upregulation of death receptor 5 overcomes the lack of p53 functionality in the diaminothiazole DAT1 induced apoptosis in colon cancer models: efficiency of DAT1 in Ras-Raf mutated cells. Mol Cancer. doi:10.1186/s12943-016-0505-7
Shoeb M, Ramana KV, Srivastava SK (2013) Aldose reductase inhibition enhances TRAIL-induced human colon cancer cell apoptosis through AKT/FOXO3a-dependent upregulation of death receptors. Free Radical Bio Med 63:280–290. doi:10.1016/j.freeradbiomed.2013.05.039
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81302172) and the China Postdoctoral Science Foundation (2013M530959 & 2014T70270). We would also like to thank Prof. KH Mayo for critical reading and editing of this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Chen, L., Meng, Y., Guo, X. et al. Gefitinib enhances human colon cancer cells to TRAIL-induced apoptosis of via autophagy- and JNK-mediated death receptors upregulation. Apoptosis 21, 1291–1301 (2016). https://doi.org/10.1007/s10495-016-1287-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-016-1287-5