Abstract
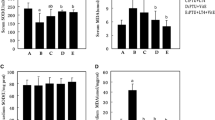
Apoptosis is a key process associated with pathological cardiac remodelling in early-phase post-myocardial infarction. In this context, several studies have demonstrated an anti-apoptotic effect of thyroid hormones (TH). The aim of this study was to evaluate the effects of TH on the expression of proteins associated with the apoptotic process 14 days after infarction. Male Wistar rats (300–350 g) (n = 8/group) were divided into four groups: Sham-operated (SHAM), infarcted (AMI), sham-operated + TH (SHAMT) and infarcted + TH (AMIT). For 12 days, the animals received T3 and T4 [2 and 8 µg/(100 g day)] by gavage. After this, the rats were submitted to haemodynamic and echocardiographic analysis, and then were sacrificed and the heart tissue was collected for molecular analysis. Statistical analyses included two-way ANOVA with Student–Newman–Keuls post test. Ethics Committee number: 23262. TH administration prevented the loss of ventricular wall thickness and improved cardiac function in the infarcted rats 14 days after the injury. AMI rats presented an increase in the pro-apoptotic proteins p53 and JNK. The hormonal treatment prevented this increase in AMIT rats. In addition, TH administration decreased the Bax:Bcl-2 ratio in the infarcted rats. TH administration improved cardiac functional parameters, and decreased the expression of pro-apoptotic proteins 14 days after myocardial infarction.




Similar content being viewed by others
References
Francis J, Weiss RM, Wei SG et al (2001) Progression of heart failure after myocardial infarction in the rat. Am J Physiol Regul Integr Comp Physiol 281:1734–1745
Jackson BM, Gorman JH, Moainie SL et al (2002) Extension of borderzone myocardium in postinfarction dilated cardiomyopathy. J Am Coll Cardiol 40:1160–1167
Abbate A, Narula J (2012) Role of apoptosis in adverse ventricular remodeling. Heart Fail Clin 8(1):79–86
Dispersyn GD, Mesotten L, Meuris B et al (2002) Dissociation of cardiomyocyte apoptosis and dedifferentiation in infarct border zones. Eur Heart J 23:849–857
Qin F, Liang MC, Liang C (2005) Progressive left ventricular remodeling, myocyte apoptosis, and protein signaling cascades after myocardial infarction in rabbits. Biochim Biophys Acta 1740:499–513
Whelan RS, Kaplinskiy V, Kitsis RN (2010) Cell death in the pathogenesis of heart disease mechanisms and significance. Annu Rev Physiol 72:19–44
Brady NR, Hamacher-Brady A, Gottlieb RA (2006) Proapoptotic BCL-2 family members and mitochondrial dysfunction during ischemia/reperfusion injury, a study employing cardiac HL-1 cells and GFP biosensors. Biochim Biophys Acta 1757:667–678
Baines CP (2010) The cardiac mitochondrion: nexus of stress. Annu Rev Physiol 72:61–80
Miyashita T, Reed J (1995) Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80:293–299
Pereg D, Cohen K, Mosseri M et al (2015) Incidence and expression of circulating cell free p53-related genes in acute myocardial infarction patients. J Atheroscler Thromb 20(1):58
Saha MN, Jiang H, Yang Y et al (2012) Targeting p53 via JNK pathway: a novel role of RITA for apoptotic signaling in multiple myeloma. PLoS One 7(1):e30215
Assaly R, de Tassigny A, Paradis S et al (2012) Oxidative stress, mitochondrial permeability transition pore opening and cell death during hypoxia-reoxygenation in adult Cardiomyocytes. Eur J Pharmacol 675:6–14
Singh N, Dhalla AK, Seneviratne C et al (1995) Oxidative stress and heart failure. Mol Cell Biochem 147(1–2):77–81
Schenkel PC, Tavares AM, Fernandes RO et al (2010) Redox-sensitive prosurvival and proapoptotic protein expression in the myocardial remodeling post-infarction in rats. Mol Cell Biochem 341:1–8
Muslin AJ (2008) MAPK signalling in cardiovascular health and disease: molecular mechanisms and therapeutic targets. Clin Sci 115(7):203–218
Forini F, Lionetti V, Ardehali H et al (2011) Early long-term L-T3 replacement rescues mitochondria and prevents ischemic cardiac remodelling in rats. J Cell Mol Med 15:514–524
Pantos C, Mourouzis I, Markakis K et al (2008) Long-term thyroid hormone administration reshapes left ventricular chamber and improves cardiac function after myocardial infarction in rats. Basic Res Cardiol 103:308–318
Mourouzis I, Giagourta I, Galanopoulos G et al (2013) Thyroid hormone improves the mechanical performance of the post-infarcted diabetic myocardium: a response associated with up-regulation of Akt/mTOR and AMPK activation. Metabolism 62:1387–1393
De Castro AL, Tavares AMV, Campos C et al (2014) Cardioprotective effects of thyroid hormones in a rat model of myocardial infarction are associated with oxidative stress reduction. Mol Cell Endocrinol 391:22–29
De Castro AL, Tavares AMV, Fernandes RO et al (2015) T3 and T4 decrease ROS levels and increase endothelial nitric oxide synthase expression in the myocardium of infarcted rats. Mol Cell Biochem. doi:10.1007/s11010-015-2501-4
Forini F, Kusmic C, Nicolini G et al (2014) Triiodothyronine prevents cardiac ischemia/reperfusion mitochondrial impairment and cell loss by regulating miR30a/p53 axis. Endocrinology 155:4581–4590
Genovese T, Impellizzeri D, Ahmad A et al (2013) Post ischaemic thyroid hormone treatment in a rat model of acute stroke. Brain Res 1513:92–102
Johns TNP, Olson BJ (1954) Experimental myocardial infarction: a method of coronary occlusion in small animals. Ann Surg 140:675–682
Nozawa E, Kanashiro RM, Murad N et al (2006) Performance of two-dimensional Doppler echocardiography for the assessment of infarct size and left ventricular function in rats. Braz J Med Biol Res 39:687–695
Peron AP, Saraiva RM, Antonio EL et al (2006) Mechanical function is normal in remanent myocardium during the healing period of myocardial infarction despite congestive heart failure. Arq Bras Cardiol 86:105–112
Tavares AM, da Rosa Araújo AS, Baldo G et al (2010) Bone marrow derived cells decrease inflammation but not oxidative stress in an experimental model of acute myocardial infarction. Life Sci 87:699–706
Araujo AS, Ribeiro MF, Enzveiler A et al (2006) Myocardial antioxidant enzyme activities and concentration and glutathione metabolism in experimental hyperthyroidism. Mol Cell Endocrinol 249:133–139
Llesuy SF, Milei J, Molina H et al (1985) Comparison of lipid peroxidation and myocardial damage induced by adramiycin and 4′-epiadramiycin in mice. Tumori 71:241–249
Lowry OH, Rosebrough NJ, Farr AL et al (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Klein D, Kern RM, Sokol RZ (1995) A method for quantification and correction of proteins after transfer to immobilization membranes. Biochem Mol Biol Int 36:59–66
Lebel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2’,7’-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5:227–231
Gonzalez Flecha B, Llesuy S, Boveris A (1991) Hydroperoxide-initiated chemiluminescence: an assay for oxidative stress in biopsies of heart, liver, and muscle. Free Radic Biol Med 10:93–100
Marklund SL (1985) Pyrogallol autooxidation. Handbook of methods for oxygen radical research CRC. Press, Boca Raton, pp 243–247
Flohe L, Gunzler WA (1984) Assays of glutathione peroxidase. Meth Enzymol 105:114–121
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Kalofoutis C, Mourouzis I, Galanopoulos G et al (2010) Thyroid hormone can favorably remodel the diabetic myocardium after acute myocardial infarction. Mol Cell Biochem 345:161–169
Grossman W, Jones D, McLaurin LP (1975) Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest 56:56–64
Pantos C, Mourouzis I, Cokkinos DV (2010) Rebuilding the post-infarcted myocardium by activating ‘physiologic’ hypertrophic signaling pathways: the thyroid hormone paradigm. Heart Fail Rev 15:143–154
Lin HY, Tang HY, Keating T et al (2008) Resveratrol is pro-apoptotic and thyroid hormone is anti-apoptotic in glioma cells: both actions are integrin and ERK mediated. Carcinogenesis 29:62–69
Yoshida K, Yoshiyama M, Omura T et al (2001) Activation of mitogen-activated protein kinases in the non-ischemic myocardium of an acute myocardial infarction in rats. Jpn Circ J 65:808–814
Yamaguchi O, Higuchi Y, Hirotani S et al (2003) Targeted deletion of apoptosis signal-regulating kinase 1 attenuates left ventricular remodeling. Proc Natl Acad Sci USA 100:15883–15888
Matsumoto-Ida M, Takimoto Y, Aoyama T et al (2006) Activation of TGF-beta1-TAK1-p38 MAPK pathway in spared cardiomyocytes is involved in left ventricular remodeling after myocardial infarction in rats. Am J Physiol Heart Circ Physiol 290:H709–H715
Fernandes RO, Bonetto JH, Baregzay B et al (2015) Modulation of apoptosis by sulforaphane is associated with PGC-1α stimulation and decreased oxidative stress in cardiac myoblasts. Mol Cell Biochem 401:61–70
Acknowledgments
This work was supported by the Brazilian Research Agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenacão de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa do Rio Grande do Sul (FAPERGS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The authors declare that the experimental procedures were performed taking into consideration the welfare of animals and were approved by the Ethics Committee for animal research at Universidade Federal do Rio Grande do Sul.
Rights and permissions
About this article
Cite this article
de Castro, A.L., Fernandes, R.O., Ortiz, V.D. et al. Thyroid hormones improve cardiac function and decrease expression of pro-apoptotic proteins in the heart of rats 14 days after infarction. Apoptosis 21, 184–194 (2016). https://doi.org/10.1007/s10495-015-1204-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-015-1204-3