Abstract
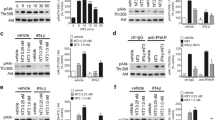
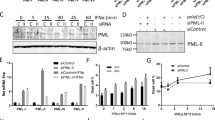
Type I interferons (IFNs) induce apoptosis of neuroblastoma cells, but the molecular mechanisms regulating this event have not been completely elucidated. Here, we investigated the role of p38 mitogen activated protein kinase (MAPK) activity, a key regulator of apoptosis and a known modulator of IFN-induced responses in non-neuronal cells. We show that in SH-SY5Y human neuroblastoma cells IFN-β induced a delayed and sustained increase of p38 MAPK activity through a novel mechanism involving the sequential activation of Janus kinase-signal transducer and activator of transcription-1 signalling, enhanced expression of the NADPH oxidase catalytic subunit gp91phox, increased reactive oxygen species production and stimulation of the MAPK kinase kinase transforming growth factor-β-activated kinase 1. Either blockade of p38 MAPK by the second generation inhibitors BIRB0796 and VX745 or siRNA knockdown of p38α MAPK enhanced IFN-β-induced apoptosis of neuroblastoma cells. Exposure to IFN-β increased the phosphorylation of the small heat shock protein HSP27 at Ser15, Ser78 and Ser82 with a time course similar to p38 MAPK activation and this response was suppressed by either p38α MAPK depletion or pharmacological inhibition of p38 MAPK and MAPK-activated protein kinase 2 (MK2). Either silencing of HSP27 expression by siRNA or MK2 inhibition potentiated IFN-β-induced apoptotic death. These results indicate that IFN-β-induced apoptosis of human SH-SY5Y neuroblastoma cells is associated with a long-lasting up-regulation of p38 MAPK activity, stimulation of MK2 and phosphorylation of the pro-survival protein HSP27. Moreover, the data show that inhibition of p38 MAPK signalling potentiates the anti-neuroblastoma activity of the cytokine, indicating that this pathway mediates a counter-regulatory response.











Similar content being viewed by others
References
Borden EC, Sen GC, Uze G, Silverman RH, Ransohoff RM, Foster G, Stark GR (2007) Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov 6:975–990
Pestka S (2007) The interferons: 50 years after their discovery, there is much more to learn. J Biol Chem 282:20047–20051
Chawla-Sarkar M, Lindner DJ, Liu Y-F, Williams BR, Sen GC, Silverman RH, Borden EC (2003) Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis 8:237–249
Kotredes KP, Gamero AM (2013) Interferons as inducers of apoptosis in malignant cells. J Interferon Cytokine Res 33:162–170
Dedoni S, Olianas MC, Onali P (2010) Interferon-β induces apoptosis in human SH-SY5Y neuroblastoma cells through activation of JAK-STAT signaling and down-regulation of PI3 K-Akt pathway. J Neurochem 115:1421–1433
Cuenda A, Rousseau S (2007) p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta 1773:1358–1375
Wagner EF, Nebreda AR (2009) Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer 9:537–549
Platanias LC (2003) The p38 mitogen-activated protein kinase pathway and its role in interferon signalling. Pharmacol Therap 98:129–142
van Boxel-Dezaire AHH, Rani MRS, Stark GR (2006) Complex modulation of cell type-specific signaling in response to type I interferons. Immunity 25:361–372
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Paul C, Manero F, Gonin S, Kretz-Remy C, Virot S, Arrigo A-P (2002) Hsp27 as a negative regulator of cytochrome c release. Mol Cell Biol 22:816–834
Dedoni S, Olianas MC, Ingianni A, Onali P (2012) Type I interferons impair BDNF-induced cell signaling and neurotrophic activity in differentiated human SH-SY5Y neuroblastoma cells and mouse primary cortical neurons. J Neurochem 122:71
de Weerd N, Samarajiwa SA, Hertzog P (2007) Type I interferon receptors: biochemistry and biological functions. J Biol Chem 282:20053–20057
Pedranzini L, Dechow T, Berishaj M, Comenzo R, Zhou P, Azare J, Bornmann W, Bromberg J (2006) Pyridone 6, a pan-Janus-activated kinase inhibitor, induces growth inhibition of multiple myeloma cells. Cancer Res 66:9714–9721
Thannickal VJ, Fanburg BL (2000) Reactive oxygen species in cell signalling. Am J Physiol Lung Cell Mol Physiol 279:L1005–L1028
McCubrey JA, Lahair MM, Franklin RA (2006) Reactive oxygen species-induced activation of the MAP kinase signaling pathway. Antiox Redox Signal 8:1775–1789
Bedard K, Krause KH (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87:245–313
Son Y, Cheong Y-K, Kim N-H, Chung H-T, Kang DG, Pae H-O (2011) Mitogen-activated protein kinases and reactive oxygen species: how can ROS activate MAPK pathways ? J Signal Transduction. doi:10.1155/2011/792639
Shirakabe K, Yamaguchi K, Shibuya H, Irie K, Matsuda S, Moriguchi T, Gotoh Y, Matsumoto K, Nishida E (1997) TAK1 mediates the ceramide signaling to stress-activated protein kinase/c-Jun N-terminal kinase. J Biol Chem 272:8141–8144
Sakurai H (2012) Targeting of TAK1 in inflammatory disorders and cancers. Trends Pharmacol Sci 33:522–530
Singhirunnusorn P, Suzuki S, Kawasaki N, Saiki I, Sakurai H (2005) Critical roles of threonine 187 phosphorylation in cellular stress-induced rapid and transient activation of transforming growth factor-β-activated kinase 1 (TAK1) in a signaling complex containing TAK1-binding protein TAB 1 and TAB 2. J Biol Chem 280:7359–7368
Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JSC, Alessi D, Cohen P (2007) The selectivity of protein kinase inhibitors: a further update. Biochem J 408:297–315
Duffy JP, Harrington EM, Salituro FG, Cochran JE, Green J, Gao H, Bemis GW, Evindar G, Galullo VP, Ford PJ, Germann UA, Wilson KP, Bellon SF, Chen G, Taslimi P, Jones P, Huang C, Pazhanisamy S, Wang Y-M, Murcko MA, Su MSS (2011) The discovery of VX-745: a novel and selective p38α kinase inhibitor. ACS Med Chem Lett 2:758–763
Guay J, Lambert H, Gingras-Breton G, Lavoie JN, Huot J, Landry J (1997) Regulation of actin filament dynamics by p38 map kinase-mediated phosphorylation of heat shock protein 27. J Cell Sci 110:357–368
Geum D, Son GH, Kim K (2002) Phosphorylation-dependent cellular localization and thermoprotective role of heat shock protein 25 in hippocampal progenitor cells. J Biol Chem 277:19913–19921
Hsu H-S, Lin J-H, Huang W-C, Hsu T-W, Su K, Chiou S-H, Tsai Y-T, Hung S-C (2011) Chemoresistance of lung cancer stemlike cells depends on activation of Hsp27. Cancer 117:1516–1528
Arrigo A-P, Simon S, Gilbert B, Kretz-Remy C, Nivon M, Czekalla A, Guillet D, Moulin M, Diaz-Latoud C, Vicart P (2007) Hsp27 (HspB1) and αB-crystallin (HspB5) as therapeutic targets. FEBS Lett 581:3665–3674
Arya R, Mallik M, Lakhotia SC (2007) Heat shock genes-Integrating cell survival and death. J Biosci 32:595–610
Pandey P, Farber R, Nakazawa A, Kumar S, Bharti A, Nalin C, Weichselbaum R, Kufe D, Kharbanda S (2000) Hsp27 functions as a negative regulator of cytochrome c-dependent activation of procaspase-3. Oncogene 19:1975–1981
Concannon CG, Orrenius S, Samali A (2001) Hsp27 inhibits cytochrome c-mediated caspase activation by sequestring both pro-caspase-3 and cytochrome c. Gene Expr 9:195–201
Bruey J-M, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, Gurbuxani S, Arrigo A-P, Kroemer G, Solary E, Garrido C (2000) Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol 2:645–652
Kostenko K, Moens U (2009) Heat shock protein 27 phosphorylation: kinases, phosphatases, functions and pathology. Cell Mol Life Sci 66:3289–3307
Huang X, Shipps GW Jr, Cheng CC, Spacciapoli P, Zhang X, McCoy MA, Wyss DF, Yang X, Achab A, Soucy K, Montavon DK, Murphy DM, Whitehurst CE (2011) Discovery and hit-to-lead optimization of non-ATP competitive MK2 (MAPKAPK2) inhibitors. ACS Med Chem Lett 2:632–637
Goh KC, Haque SJ, Williams BRG (1999) p38 MAP kinase is required for STAT1 serine phosphorylation and transcriptional activation induced by interferons. EMBO J 18:5601–5608
Uddin S, Majchrzak B, Woodson J, Arunkumar P, Alsayed Y, Pine R, Young PR, Fish EN, Platanias LC (1999) Activation of the p38 mitogen-activated protein kinase by type I interferons. J Biol Chem 274:30127–30131
Luo W, Skalnik DG (1996) Interferon regulatory factor-2 directs transcription from the gp91phox promoter. J Biol Chem 271:23445–23451
Eklund EA, Jalava A, Kakar R (1998) PU.1, interferon regulatory factor 1, and interferon consensus sequence-binding protein cooperate to increase gp91phox expression. J Biol Chem 273:13957–13965
Kumatori A, Yang D, Suzuki S, Nakamura M (2002) Cooperation of STAT-1 and IRF-1 in interferon-γ-induced transcription of the gp91phox gene. J Biol Chem 277:9103–9111
Kuwano Y, Kawahara T, Yamamoto H, Teshima-Kondo S, Tominaga K, Masuda K, Kishi K, Morita K, Rokutan K (2006) Interferon-γ activates transcription of NADPH oxidase I gene and upregulates production of superoxide anion by human large intestinal epithelial cells. Am J Physiol Cell Physiol 290:C433–C443
Ninomiya-Tsuji J, Kajino T, Ono K, Ohtomo T, Matsumoto M, Shiina M, Mihara M, Tsuchiya M, Matsumoto K (2003) A resorcylic acid lactone, 5Z-7-oxozeaenol, prevents inflammation by inhibiting the catalytic activity of TAK1 MAPK kinase kinase. J Biol Chem 278:18485–18490
Li MG, Katsura K, Moniyama H, Komaki K, Ninomiya-Tsuji J, Matsumoto K, Kobayashi T, Tamura S (2003) Regulation of the interleukin-1-induced signaling pathways by a novel member of the protein phosphatase 2C family (PP2Cε). J Biol Chem 278:12013–12021
Kim S II, Kwak JH, Wang L, Choi ME (2008) Protein phosphatase 2A is a negative regulator of transforming growth factor-β1-induced TAK1 activation in mesangial cells. J Biol Chem 283:10753–10763
Chen L, Liu L, Yin J, Luo Y, Huang S (2009) Hydrogen peroxide-induced neuronal apoptosis is associated with inhibition of protein phosphatase 2A and 5, leading to activation of MAPK pathway. Int J Biochem Cell Biol 41:1284–1295
Ghatan S, Larner S, Kinoshita Y, Hetman M, Patel L, Xia Z, Youle RJ, Morrison RS (2000) p38 MAP kinase mediates bax translocation in nitric oxide-induced apoptosis in neurons. J Cell Biol 150:335–347
Lu J, Xu SY, Zhang QG, Xu R, Lei HY (2011) Bupivacaine induces apoptosis via mitochondria and p38 MAPK dependent pathways. Eur J Pharmacol 657:51–58
Filomeni G, Piccirillo S, Rotilio G, Ciriolo MR (2012) p38 MAPK and ERK1/2 dictate cell death/survival response to different pro-oxidant stimuli via p53 and Nrf2 in neuroblastoma cells SH-SY5Y. Biochem Pharmacol 83:1349–1357
Nemoto S, Xiang J, Huang S, Lin A (1998) Induction of apoptosis by SB202190 through inhibition of p38β mitogen-activated protein kinase. J Biol Chem 273:16415–16420
Kuma Y, Sabio G, Bain J, Shpiro N, Marquez R, Cuenda A (2005) BIRB796 inhibits all p38 MAPK isoforms in vitro and in vivo. J Biol Chem 280:19472–19479
Wei L, Liu T-T, Wang H-H, Hong H-M, Yu AL, Feng H-P, Chang WW (2011) Hsp27 participates in the maintenance of breast cancer stem cells through regulation of epithelial-mesenchymal transition and nuclear factor-κB. Breast Cancer Res 13:R101
Shin KD, Lee M-Y, Shin D-S, Lee S, Son K-H, Koh S, Paik Y-K, Kwon B-M, Han DC (2005) Blocking tumor cell migration and invasion with biphenyl isoxazole derivative KRIGG3, a synthetic molecule that inhibits Hsp27 phosphorylation. J Biol Chem 280:41439–41448
Huot J, Houle F, Spitz DR, Landry J (1996) Hsp27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress. Cancer Res 56:273–279
Benndorf R, Hayeß K, Ryazantsev S, Wieske M, Behlke J, Lutsch G (1994) Phosphorylation and supramolecular organization of murine small heat shock protein HSP abolish its actin polymerization-inhibiting activity. J Biol Chem 269:20780–20784
Lavoie JN, Lambert H, Hickey E, Weber LA, Landry J (1995) Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27. Mol Cell Biol 15:505–516
Mielke K, Damm A, Yang DD, Herdegen T (2000) Selective expression of JNK isoforms and stress-specific JNK activity in different neural cell lines. Mol Brain Res 75:128–137
Denny JB (2012) The cell biology of neuroblastoma. In: Shimada H (ed) Neuroblastoma-present and future. InTech pp. 55–78
Pistoia V, Bianchi G, Borgonovo G, Raffaghello L (2011) Cytokines in neuroblastoma: from pathogenesis to treatment. Immunotherapy 3:895–907
Chiang J, Gloff CA, Yoshizawa CN, Williams GJ (1993) Pharmacokinetics of recombinant human interferon-beta ser in healthy volunteers and its effect on serum neopterin. Pharm Res 10:567–572
Acknowledgments
The study was supported by Regione Autonoma della Sardegna, L.R. n.7/2007- CRP 10810/2012 and MIUR D.M.28142.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dedoni, S., Olianas, M.C. & Onali, P. Interferon-β counter-regulates its own pro-apoptotic action by activating p38 MAPK signalling in human SH-SY5Y neuroblastoma cells. Apoptosis 19, 1509–1526 (2014). https://doi.org/10.1007/s10495-014-1024-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-014-1024-x