Abstract
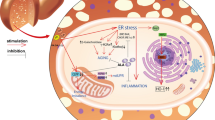
A high plasma concentration of non-esterified fatty acids (NEFAs) is an important pathogenic factor that leads to ketosis and fatty liver in dairy cows. NEFAs may be associated with oxidative stress in dairy cows with ketosis or fatty liver and the subsequent induction of hepatocyte damage. However, the molecular mechanism of NEFAs-induced oxidative stress and whether NEFAs cause apoptosis of hepatocytes are unclear. Therefore, the aim of this study was to investigate the molecular mechanism of NEFAs-induced oxidative liver damage in bovine hepatocytes. The results showed that NEFAs increased oxidative stress, resulting in p38 phosphorylation. High activated p38 increased the expression, nuclear localization and transcriptional activity of p53 and decreased the nuclear localization and transcriptional activity of Nrf2 in bovine hepatocytes treated with high concentrations of NEFAs. High concentrations of NEFAs also promoted the apoptosis of bovine hepatocytes. Both N-acetyl-l-cysteine (NAC) and glucose (GLU) could attenuate the NEFA-induced apoptotic damage. These results indicate that NEFAs activate the ROS–p38–p53/Nrf2 signaling pathway to induce apoptotic damage in bovine hepatocytes.









Similar content being viewed by others
Abbreviations
- BSA:
-
Bovine serum albumin
- CAT:
-
Catalase
- GLU:
-
Glucose
- GSH:
-
Glutathione
- GSSG:
-
Glutathione disulfide
- GSH-Px:
-
Glutathione peroxidase
- Keap1:
-
Kelch-like ECH-associated protein 1
- MDA:
-
Malonaldehyde
- Mdm2:
-
Mouse double minute 2 homolog
- NAC:
-
N-acetyl-l-cysteine
- NEB:
-
Negative energy balance
- NEFA:
-
Non-esterified fatty acids
- Nrf2:
-
Nuclear factor erythroid 2-related factor2
- p38MAPK:
-
p38 mitogen-activated protein kinases
- p53:
-
Tumor protein p53
- RET:
-
Reverse electron transfer
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TAC:
-
Total antioxidant capacity
References
Wathes DC, Cheng Z, Fenwick MA, Fitzpatrick R, Patton J (2011) Influence of energy balance on the somatotrophic axis and matrix metalloproteinase expression in the endometrium of the postpartum dairy cow. Reproduction 141:269–281
Loor JJ, Everts RE, Bionaz M, Dann HM, Morin DE, Oliveira R et al (2007) Nutrition-induced ketosis alters metabolic and signaling gene networks in liver of periparturient dairy cows. Physiol Genomics 32:105–116
Herdt TH (2000) Ruminant adaptation to negative energy balance. Influences on the etiology of ketosis and fatty liver. Vet Clin North Am Food Anim Pract 16:215–230
Jorritsma R, Jorritsma H, Schukken YH, Bartlett PC, Wensing T, Wentink GH (2001) Prevalence and indicators of post partum fatty infiltration of the liver in nine commercial dairy herds in The Netherlands. Livest Prod Sci 68:53–60
Adewuyi AA, Gruys E, van Eerdenburg FJCM (2005) Non esterified fatty acids (NEFA) in dairy cattle. A review. Vet Q 27:117–126
Contreras GA, Sordillo LM (2011) Lipid mobilization and inflammatory responses during the transition period of dairy cows. Comp Immunol Microbiol Infect Dis 34:281–289
Schonfeld P, Wojtczak L (2008) Fatty acids as modulators of the cellular production of reactive oxygen species. Free Radic Biol Med 45:231–241
Leclercq GRI, Farrell GC (2001) Nonalcoholic steatosis and steatohepatitis—II. Cytochrome P-450 enzymes and oxidative stress. Am J Physiol Gastrointest Liver Physiol 281:G1135–G1139
Hufnagel B, Dworak M, Soufi M, Mester Z, Zhu Y, Schaefer JR et al (2005) Unsaturated fatty acids isolated from human lipoproteins activate protein phosphatase type 2C beta and induce apoptosis in endothelial cells. Atherosclerosis 180:245–254
Cnop M (2008) Fatty acids and glucolipotoxicity in the pathogenesis of type 2 diabetes. Biochem Soc Trans 36:348–352
Seko Y, Takahashi N, Tobe K, Kadowaki T, Yazaki Y (1997) Hypoxia and hypoxia/reoxygenation activate p65PAK, p38 mitogen-activated protein kinase (MAPK), and stress-activated protein kinase (SAPK) in cultured rat cardiac myocytes. Biochem Biophys Res Commun 239:840–844
Tormos AM, Talens-Visconti R, Nebreda AR, Sastre J (2013) p38 MAPK: a dual role in hepatocyte proliferation through reactive oxygen species. Free Radic Res 47:905–916
Circu ML, Aw TY (2010) Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med 48:749–762
Hwang YP, Jeong HG (2008) The coffee diterpene kahweol induces heme oxygenase-1 via the PI3K and p38/Nrf2 pathway to protect human dopaminergic neurons from 6-hydroxydopamine-derived oxidative stress. FEBS Lett 582:2655–2662
Liu B, Cheng Y, Zhang B, Bian HJ, Bao JK (2009) Polygonatum cyrtonema lectin induces apoptosis and autophagy in human melanoma A375 cells through a mitochondria-mediated ROS–p38–p53 pathway. Cancer Lett 275:54–60
Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM (1990) The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63:1129–1136
She QB, Chen NY, Dong ZG (2000) ERKs and p38 kinase phosphorylate p53 protein at serine 15 in response to UV radiation. J Biol Chem 275:20444–20449
Yuan HP, Zhang XY, Huang XQ, Lu YG, Tang WQ, Man Y et al (2010) NADPH oxidase 2-derived reactive oxygen species mediate FFAs-induced dysfunction and apoptosis of beta-cells via JNK, p38 MAPK and p53 pathways. PLoS One 5:e15726
Panasiuk A, Dzieciol J, Panasiuk B, Prokopowicz D (2006) Expression of p53, Bax and Bcl-2 proteins in hepatocytes in non-alcoholic fatty liver disease. World J Gastroenterol 12:6198–6202
Jaiswal AK (2004) Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med 36:1199–1207
Zhang ZG, Li XB, Gao L, Liu GW, Kong T, Li YF et al (2012) An updated method for the isolation and culture of primary calf hepatocytes. Vet J 191:323–326
Ingvartsen KL, Andersen JB (2000) Integration of metabolism and intake regulation: a review focusing on periparturient animals. J Dairy Sci 83:1573–1597
Drackley JK (1999) Biology of dairy cows during the transition period: the final frontier? J Dairy Sci 82:2259–2273
Oikawa S, Mizunuma Y, Iwasaki Y, Tharwat M (2010) Changes of very low-density lipoprotein concentration in hepatic blood from cows with fasting-induced hepatic lipidosis. Can J Vet Res 74:317–320
Walsh RB, Walton JS, Kelton DF, LeBlanc SJ, Leslie KE, Duffield TF (2007) The effect of subclinical ketosis in early lactation on reproductive performance of postpartum dairy cows. J Dairy Sci 90:2788–2796
Cnop M, Igoillo-Esteve M, Cunha DA, Ladriere L, Eizirik DL (2008) An update on lipotoxic endoplasmic reticulum stress in pancreatic beta-cells. Biochem Soc Trans 36:909–915
Boden G (2008) Obesity and free fatty acids. Endocrinol Metab Clin North Am 37:635–646
Cardone MH, Salvesen GS, Widmann C, Johnson G, Frisch SM (1997) The regulation of anoikis: MEKK-1 activation requires cleavage by caspases. Cell 90:315–323
Zarubin T, Han J (2005) Activation and signaling of the p38 MAP kinase pathway. Cell Res 15:11–18
Lavin MF, Gueven N (2006) The complexity of p53 stabilization and activation. Cell Death Differ 13:941–950
Wei CL, Wu Q, Vega VB, Chiu KP, Ng P, Zhang T et al (2006) A global map of p53 transcription-factor binding sites in the human genome. Cell 124:207–219
Hayes JD, McMahon M (2009) NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci 34:176–188
Martin D, Rojo AI, Salinas M, Diaz R, Gallardo G, Alam J et al (2004) Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J Biol Chem 279:8919–8929
Li HY, Wu SY, Shi NA, Lian SQ, Lin W (2011) Nrf2/HO-1 pathway activation by manganese is associated with reactive oxygen species and ubiquitin–proteasome pathway, not MAPKs signaling. J Appl Toxicol 31:690–697
Shen GX, Hebbar V, Nair S, Xu CJ, Li WG, Lin W et al (2004) Regulation of Nrf2 transactivation domain activity—the differential effects of mitogen-activated protein kinase cascades and synergistic stimulatory effect of Raf and CREB-binding protein. J Biol Chem 279:23052–23060
Faraonio R, Vergara P, Di Marzo D, Pierantoni MG, Napolitano M, Russo T et al (2006) p53 suppresses the Nrf2-dependent transcription of antioxidant response genes. J Biol Chem 281:39776–39784
Acknowledgments
This work was supported by the National High Technology R&D Program (No. 2013AA102806), the National Key Technology R&D Program (No. 2012BAD12B03), the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT, No. IRT1248), the National Natural Science Foundation of China (Beijing, China; Grant No. 30871897, 30972224, 31072178, 31172372, 31272621, 31360630, 31372478 and 31372494), the Program for New Century Excellent Talents in University (NCET-11-0199).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yuxiang Song and Xinwei Li contributed equally to this study.
Rights and permissions
About this article
Cite this article
Song, Y., Li, X., Li, Y. et al. Non-esterified fatty acids activate the ROS–p38–p53/Nrf2 signaling pathway to induce bovine hepatocyte apoptosis in vitro. Apoptosis 19, 984–997 (2014). https://doi.org/10.1007/s10495-014-0982-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-014-0982-3