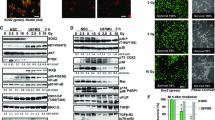
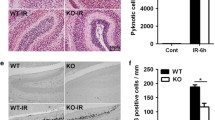
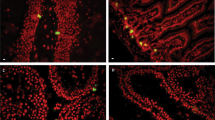
Abstract
Adult neurons, which are terminally differentiated cells, demonstrate substantial radioresistance. In contrast, human neural stem cells (NSC), which have a significant proliferative capacity, are highly sensitive to ionizing radiation. Cranial irradiation that is widely used for treatment of brain tumors may induce death of NSC and further cause substantial cognitive deficits such as impairing learning and memory. The main goal of our study was to determine a mechanism of NSC radiosensitivity. We observed a constitutive high-level expression of TRAIL-R2 in human NSC. On the other hand, ionizing radiation through generation of reactive oxygen species targeted cell signaling pathways and dramatically changed the pattern of gene expression, including upregulation of TRAIL. A significant increase of endogenous expression and secretion of TRAIL could induce autocrine/paracrine stimulation of the TRAIL-R2-mediated signaling cascade with activation of caspase-3-driven apoptosis. Furthermore, paracrine stimulation could initiate bystander response of non-targeted NSC that is driven by death ligands produced by directly irradiated NSC. Experiments with media transfer from directly irradiated NSC to non-targeted (bystander) NSC confirmed a role of secreted TRAIL for induction of a death signaling cascade in non-targeted NSC. Subsequently, TRAIL production through elimination of bystander TRAIL-R-positive NSC might substantially restrict a final yield of differentiating young neurons. Radiation-induced TRAIL-mediated apoptosis could be partially suppressed by anti-TRAIL antibody added to the cell media. Interestingly, direct gamma-irradiation of SK-N-SH human neuroblastoma cells using clinical doses (2–5 Gy) resulted in low levels of apoptosis in cancer cells that was accompanied however by induction of a strong bystander response in non-targeted NSC. Numerous protective mechanisms were involved in the maintenance of radioresistance of neuroblastoma cells, including constitutive PI3K-AKT over-activation and endogenous synthesis of TGFβ1. Specific blockage of these survival pathways was accompanied by a dramatic increase in radiosensitivity of neuroblastoma cells. Intercellular communication between cancer cells and NSC could potentially be involved in amplification of cancer pathology in the brain.










Similar content being viewed by others
Abbreviations
- FACS:
-
Fluorescence-activated cell sorter
- FGF2:
-
Fibroblast growth factor-2 (basic)
- DR5:
-
Death receptor-5 (synonym for TRAIL-R2)
- IκB:
-
Inhibitor of NF-κB
- IKK:
-
Inhibitor nuclear factor kappa B kinase
- JNK:
-
c-Jun N-terminal kinase
- MAPK:
-
Mitogen-activated protein kinase
- MEK:
-
MAPK/ERK kinase
- MEF:
-
Median fluorescence intensity
- NF-κB:
-
Nuclear factor kappa B
- NSC:
-
Neural stem cells
- PARP1:
-
Poly (ADP-ribose) polymerase-1
- PI:
-
Propidium iodide
- STAT:
-
Signal transducers and activators of transcription
- TGFβ:
-
Transforming growth factor beta
- TGFβ-R:
-
TGFβ-receptor
- TNFα:
-
Tumor necrosis factor alpha
- TRAIL:
-
TNF-related apoptosis ligand
- TRAIL-R:
-
TRAIL-receptor
- zVAD:
-
Carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone
References
Monje ML, Mizumatsu S, Fike JR, Palmer TD (2002) Irradiation induces neural precursor-cell dysfunction. Nat Med 8:955–962
Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR (2003) Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res 63:4021–4027
Acharya MM, Lan ML, Kan VH, Patel NH, Giedzinski E, Tseng BP, Limoli CL (2010) Consequences of ionizing radiation-induced damage in human neural stem cells. Free Radic Biol Med 49:1846–1855
Acharya MM, Christie LA, Lan ML, Giedzinski E, Fike JR, Rosi S, Limoli CL (2011) Human neural stem cell transplantation ameliorates radiation-induced cognitive dysfunction. Cancer Res 71:4834–4845
Hellstrom NA, Bjork-Eriksson T, Blomgren K, Kuhn HG (2009) Differential recovery of neural stem cells in the subventricular zone and dentate gyrus after ionizing radiation. Stem Cells 27:634–641
Greene-Schloesser D, Robbins ME, Peiffer AM, Shaw EG, Wheeler KT, Chan MD (2012) Radiation-induced brain injury: a review. Front Oncol 2:73
Okada H, Mak TW (2004) Pathways of apoptotic and non-apoptotic death in tumour cells. Nat Rev Cancer 4:592–603
Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ, Yuan J (2008) Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell 135:1311–1323
Hei TK, Zhou H, Chai Y, Ponnaiya B, Ivanov VN (2011) Radiation induced non-targeted response: mechanism and potential clinical implications. Curr Mol Pharmacol 4:96–105
Ivanov VN, Ghandhi SA, Zhou H, Huang SX, Chai Y, Amundson SA, Hei TK (2011) Radiation response and regulation of apoptosis induced by a combination of TRAIL and CHX in cells lacking mitochondrial DNA: a role for NF-kappaB-STAT3-directed gene expression. Exp Cell Res 317:1548–1566
Ivanov VN, Zhou H, Ghandhi SA, Karasic TB, Yaghoubian B, Amundson SA, Hei TK (2010) Radiation-induced bystander signaling pathways in human fibroblasts: a role for interleukin-33 in the signal transmission. Cell Signal 22:1076–1087
Prise KM, O’Sullivan JM (2009) Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer 9:351–360
Morgan WF (2003) Non-targeted and delayed effects of exposure to ionizing radiation: I. Radiation-induced genomic instability and bystander effects in vitro. Radiat Res 159:567–580
Zhao C, Deng W, Gage FH (2008) Mechanisms and functional implications of adult neurogenesis. Cell 132:645–660
Allen JE, El-Deiry WS (2012) Regulation of the human TRAIL gene. Cancer Biol Ther 13:1143–1151
Owen-Schaub LB, Zhang W, Cusack JC, Angelo LS, Santee SM, Fujiwara T, Roth JA, Deisseroth AB, Zhang WW, Kruzel E et al (1995) Wild-type human p53 and a temperature-sensitive mutant induce Fas/APO-1 expression. Mol Cell Biol 15:3032–3040
Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, Hedrick SM, Gerber SA, Lugovskoy A, Yuan J (2008) Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol 4:313–321
Block ML, Zecca L, Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8:57–69
Haase G, Pettmann B, Raoul C, Henderson CE (2008) Signaling by death receptors in the nervous system. Curr Opin Neurobiol 18:284–291
Song JH, Bellail A, Tse MC, Yong VW, Hao C (2006) Human astrocytes are resistant to Fas ligand and tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis. J Neurosci 26:3299–3308
Schneider L, Fumagalli M, d’Adda di Fagagna F (2012) Terminally differentiated astrocytes lack DNA damage response signaling and are radioresistant but retain DNA repair proficiency. Cell Death Differ 19:582–591
Cheung NK, Dyer MA (2013) Neuroblastoma: developmental biology, cancer genomics and immunotherapy. Nat Rev Cancer 13:397–411
Jaing TH, Yang CP, Hung IJ, Wang HS, Tseng CK, Hsueh C (2003) Brain metastases in children with neuroblastoma–a single-institution experience. Med Pediatr Oncol 41:570–571
Ivanov VN, Hei TK (2011) Regulation of apoptosis in human melanoma and neuroblastoma cells by statins, sodium arsenite and TRAIL: a role of combined treatment versus monotherapy. Apoptosis 16:1268–1284
Johnsen JI, Lindskog M, Ponthan F, Pettersen I, Elfman L, Orrego A, Sveinbjornsson B, Kogner P (2004) Cyclooxygenase-2 is expressed in neuroblastoma, and nonsteroidal anti-inflammatory drugs induce apoptosis and inhibit tumor growth in vivo. Cancer Res 64:7210–7215
Rasmuson A, Kock A, Fuskevag OM, Kruspig B, Simon-Santamaria J, Gogvadze V, Johnsen JI, Kogner P, Sveinbjornsson B (2012) Autocrine prostaglandin E2 signaling promotes tumor cell survival and proliferation in childhood neuroblastoma. PLoS One 7:e29331
Castriconi R, Dondero A, Bellora F, Moretta L, Castellano A, Locatelli F, Corrias MV, Moretta A, Bottino C (2013) Neuroblastoma-derived TGF-beta1 modulates the chemokine receptor repertoire of human resting NK cells. J Immunol 190:5321–5328
Hardee ME, Marciscano AE, Medina-Ramirez CM, Zagzag D, Narayana A, Lonning SM, Barcellos-Hoff MH (2012) Resistance of glioblastoma-initiating cells to radiation mediated by the tumor microenvironment can be abolished by inhibiting transforming growth factor-beta. Cancer Res 72:4119–4129
Ashkenazi A, Holland P, Eckhardt SG (2008) Ligand-based targeting of apoptosis in cancer: the potential of recombinant human apoptosis ligand 2/Tumor necrosis factor-related apoptosis-inducing ligand (rhApo2L/TRAIL). J Clin Oncol 26:3621–3630
Finnberg N, Klein-Szanto AJ, El-Deiry WS (2008) TRAIL-R deficiency in mice promotes susceptibility to chronic inflammation and tumorigenesis. J Clin Invest 118:111–123
Adams C, Totpal K, Lawrence D, Marsters S, Pitti R, Yee S, Ross S, Deforge L, Koeppen H, Sagolla M, Compaan D, Lowman H, Hymowitz S, Ashkenazi A (2008) Structural and functional analysis of the interaction between the agonistic monoclonal antibody Apomab and the proapoptotic receptor DR5. Cell Death Differ 15:751–761
Balyasnikova IV, Ferguson SD, Han Y, Liu F, Lesniak MS (2011) Therapeutic effect of neural stem cells expressing TRAIL and bortezomib in mice with glioma xenografts. Cancer Lett 310:148–159
Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J (2005) Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 1:112–119
Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G (2010) Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 11:700–714
Karin M (2009) NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol 1:a000141
Walczak H (2013) Death receptor-ligand systems in cancer, cell death, and inflammation. Cold Spring Harb Perspect Biol 5:a008698
Knight JC, Scharf EL, Mao-Draayer Y (2010) Fas activation increases neural progenitor cell survival. J Neurosci Res 88:746–757
Ghandhi SA, Ming L, Ivanov VN, Hei TK, Amundson SA (2010) Regulation of early signaling and gene expression in the alpha-particle and bystander response of IMR-90 human fibroblasts. BMC Med Genomics 3:31
Ivanov VN, Ghandhi SA, Zhou H, Huang SX, Chai Y, Amundson SA, Hei TK (2011) Radiation response and regulation of apoptosis induced by a combination of TRAIL and CHX in cells lacking mitochondrial DNA: a role for NF-[kappa]B-STAT3-directed gene expression. Exp Cell Res 317:1548–1566
Zhou H, Ivanov VN, Lien YC, Davidson M, Hei TK (2008) Mitochondrial function and nuclear factor-kappaB-mediated signaling in radiation-induced bystander effects. Cancer Res 68:2233–2240
Ivanov VN, Hei TK (2006) Dual treatment with COX-2 inhibitor and sodium arsenite leads to induction of surface Fas Ligand expression and Fas-Ligand-mediated apoptosis in human melanoma cells. Exp Cell Res 312:1401–1417
Zhou H, Ivanov VN, Gillespie J, Geard CR, Amundson SA, Brenner DJ, Yu Z, Lieberman HB, Hei TK (2005) Mechanism of radiation-induced bystander effect: role of the cyclooxygenase-2 signaling pathway. Proc Natl Acad Sci USA 102:14641–14646
Ivanov VN, Hei TK (2013) Induction of apoptotic death and retardation of neuronal differentiation of human neural stem cells by sodium arsenite treatment. Exp Cell Res 319:875–887
Acknowledgments
We would like to thank Drs. Adayabalam Balajee, Mercy Davidson, Peter Grabham and Howard Lieberman for advice, critical reading of the manuscript and discussion. This work was supported by NIH Grant P01 CA049062 and Pilot Grant of the Department of Dermatology, Columbia University (P30AR044531-11, Project GG006336).
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ivanov, V.N., Hei, T.K. A role for TRAIL/TRAIL-R2 in radiation-induced apoptosis and radiation-induced bystander response of human neural stem cells. Apoptosis 19, 399–413 (2014). https://doi.org/10.1007/s10495-013-0925-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-013-0925-4