Abstract
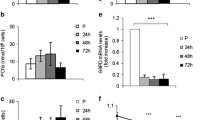
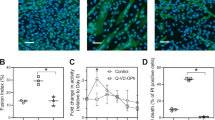
Muscle cell apoptosis accompanies normal muscle development and regeneration, as well as degenerative diseases and aging. C2C12 murine myoblast cells represent a common model to study muscle differentiation. Though it was already shown that myogenic differentiation of C2C12 cells is accompanied by enhanced apoptosis in a fraction of cells, either the cell population sensitive to apoptosis or regulatory mechanisms for the apoptotic response are unclear so far. In the current study we characterize apoptotic phenotypes of different types of C2C12 cells at all stages of differentiation, and report here that myotubes of differentiated C2C12 cells with low levels of anti-apoptotic Bcl-2 expression are particularly vulnerable to apoptosis even though they are displaying low levels of pro-apoptotic proteins Bax, Bak and Bad. In contrast, reserve cells exhibit higher levels of Bcl-2 and high resistance to apoptosis. The transfection of proliferating myoblasts with Bcl-2 prior to differentiation did not protect against spontaneous apoptosis accompanying differentiation of C2C12 cells but led to Bcl-2 overexpression in myotubes and to significant protection from apoptotic cell loss caused by exposure to hydrogen peroxide. Overall, our data advocate for a Bcl-2-dependent mechanism of apoptosis in differentiated muscle cells. However, downstream processes for spontaneous and hydrogen peroxide induced apoptosis are not completely similar. Apoptosis in differentiating myoblasts and myotubes is regulated not through interaction of Bcl-2 with pro-apoptotic Bcl-2 family proteins such as Bax, Bak, and Bad.










Similar content being viewed by others
Abbreviations
- DM:
-
Differentiation medium
- ER:
-
Endoplasmic reticulum
- GM:
-
Growing medium
- TG:
-
Thapsigargin
- TIC:
-
Total ion current
References
Matsuda R, Nishikawa A, Tanaka H (1995) Visualization of dystrophic muscle fibers in mdx mouse by vital staining with Evans blue: evidence of apoptosis in dystrophin-deficient muscle. J Biochem 118(5):959–964
McClearn D, Medville R, Noden D (1995) Muscle cell death during the development of head and neck muscles in the chick embryo. Dev Dyn 202(4):365–377
Wang J, Walsh K (1996) Resistance to apoptosis conferred by Cdk inhibitors during myocyte differentiation. Science 273(5273):359–361
Dominov JA, Kravetz AJ, Ardelt M, Kostek CA, Beermann ML, Miller JB (2005) Muscle-specific BCL2 expression ameliorates muscle disease in laminin {alpha}2-deficient, but not in dystrophin-deficient, mice. Hum Mol Genet 14(8):1029–1040
Baker DJ, Hepple RT (2006) Elevated caspase and AIF gene expression correlate with progression of sarcopenia during aging in male F344BN rats. Exp Gerontol 41(11):1149–1156
Bejma J, Ji LL (1999) Aging and acute exercise enhance free radical generation in rat skeletal muscle. J Appl Physiol 87(1):465–470
Alway SE, Degens H, Krishnamurthy G, Chaudhrai A (2003) Denervation stimulates apoptosis but not Id2 expression in hindlimb muscles of aged rats. J Gerontol A 58(8):687–697
Cortopassi GA, Wong A (1999) Mitochondria in organismal aging and degeneration. Biochim Biophys Acta 1410(2):183–193
Chabi B, Ljubicic V, Menzies KJ, Huang JH, Saleem A, Hood DA (2008) Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell 7(1):2–12
Dirks AJ, Leeuwenburgh C (2005) The role of apoptosis in age-related skeletal muscle atrophy. Sports Med 35(6):473–483
Whitman SA, Wacker MJ, Richmond SR, Godard MP (2005) Contributions of the ubiquitin-proteasome pathway and apoptosis to human skeletal muscle wasting with age. Pflugers Arch 450(6):437–446
Thornberry NA, Lazebnik Y (1998) Caspases: enemies within. Science 281(5381):1312–1316
Murray TV, McMahon JM, Howley BA, Stanley A, Ritter T, Mohr A, Zwacka R, Fearnhead HO (2008) A non-apoptotic role for caspase-9 in muscle differentiation. J Cell Sci 121(Pt 22):3786–3793
Huppertz B, Tews DS, Kaufmann P (2001) Apoptosis and syncytial fusion in human placental trophoblast and skeletal muscle. Int Rev Cytol 205:215–253
Nakanishi K, Sudo T, Morishima N (2005) Endoplasmic reticulum stress signaling transmitted by ATF6 mediates apoptosis during muscle development. J Cell Biol 169(4):555–560
Nakanishi K, Dohmae N, Morishima N (2007) Endoplasmic reticulum stress increases myofiber formation in vitro. FASEB J 21(11):2994–3003
Davies JE, Rubinsztein DC (2011) Over-expression of BCL2 rescues muscle weakness in a mouse model of oculopharyngeal muscular dystrophy. Hum Mol Genet 20(6):1154–1163
Siu PM, Bryner RW, Martyn JK, Alway SE (2004) Apoptotic adaptations from exercise training in skeletal and cardiac muscles. FASEB J 18(10):1150–1152
Jejurikar SS, Henkelman EA, Cederna PS, Marcelo CL, Urbanchek MG, Kuzon WM Jr (2006) Aging increases the susceptibility of skeletal muscle derived satellite cells to apoptosis. Exp Gerontol 41(9):828–836
Siu PM, Wang Y, Alway SE (2009) Apoptotic signaling induced by H2O2-mediated oxidative stress in differentiated C2C12 myotubes. Life Sci 84(13–14):468–481
Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD (1997) The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275(5303):1132–1136
Gross A, McDonnell JM, Korsmeyer SJ (1999) BCL-2 family members and the mitochondria in apoptosis. Genes Dev 13(15):1899–1911
Ma TS, Mann DL, Lee JH, Gallinghouse G (1999) SR compartment calcium and cell apoptosis in SERCA overexpression. Cell Calcium 26(1–2):25–36
Patterson RL, Boehning D, Snyder SH (2004) Inositol-1,4,5-trisphosphate receptors as signal integrators. Annu Rev Biochem 73:437–465
Hajnóczky G, Davies E, Madesh M (2003) Calcium signaling and apoptosis. Biochem Biophys Res Commun 304(3):445–454
Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R (2008) Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 27(50):6407–6418
Rodriguez D, Rojas-Rivera D (1813) Hetz C (2011) Integrating stress signals at the endoplasmic reticulum: the Bcl-2 protein family rheostat. Biochim Biophys Acta 4:564–574
Gailly P (2002) New aspects of calcium signaling in skeletal muscle cells: implications in Duchenne muscular dystrophy. Biochim Biophys Acta 1600(1–2):38–44
Imbert N, Cognard C, Duport G, Guillou C, Raymond G (1995) Abnormal calcium homeostasis in Duchenne muscular dystrophy myotubes contracting in vitro. Cell Calcium 18(3):177–186
Bianchi K, Rimessi A, Prandini A, Szabadkai G, Rizzuto R (2004) Calcium and mitochondria: mechanisms and functions of a troubled relationship. Biochim Biophys Acta 1742(1–3):119–131
Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T (1998) Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280(5370):1763–1766
Basset O, Boittin FX, Cognard C, Constantin B, Ruegg UT (2006) Bcl-2 overexpression prevents calcium overload and subsequent apoptosis in dystrophic myotubes. Biochem J 395(2):267–276
Dremina ES, Sharov VS, Kumar K, Zaidi A, Michaelis EK, Schöneich C (2004) Anti-apoptotic protein Bcl-2 interacts with and destabilizes the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA). Biochem J 383((Pt2)):361–370
Dremina ES, Sharov VS, Schöneich C (2006) Displacement of SERCA from SR lipid caveolae-related domains by Bcl-2: a possible mechanism for SERCA inactivation. Biochemistry 45(1):175–184
Dremina ES, Sharov VS, Schöneich C (2012) Heat shock proteins attenuate SERCA inactivation by the anti-apoptotic protein Bcl-2: possible implications for the ER Ca2+ mediated apoptosis. Biochem J 444(1):127–139
Dominov JA, Dunn JJ, Miller JB (1998) Bcl-2 expression identifies an early stage of myogenesis and promotes clonal expansion of muscle cells. J Cell Biol 1429(2):537–544
Dominov JA, Houlihan-Kawamoto CA, Swap CJ, Miller JB (2001) Pro- and anti-apoptotic members of the Bcl-2 family in skeletal muscle: a distinct role for Bcl-2 in later stages of myogenesis. Dev Dyn 220(1):18–26
Kitzmann M, Carnac G, Vandromme M, Primig M, Lamb NJ, Fernandez A (1998) The muscle regulatory factors MyoD and myf-5 undergo distinct cell cycle-specific expression in muscle cells. J Cell Biol 142(6):1447–1459
Dally S, Bredoux R, Corvazier E, Andersen JP, Clausen JD, Dode L, Fanchaouy M, Gelebart P, Monceau V, Del Monte F, Gwathmey JK, Hajjar R, Chaabane C, Bobe R, Raies A, Enouf J (2006) Ca2+ -ATPases in non-failing and failing heart: evidence for a novel cardiac sarco/endoplasmic reticulum Ca2+ -ATPase 2 isoform (SERCA2c). Biochem J 395(2):249–258
Olsen JV, Mann M (2004) Improved peptide identification in proteomics by two consecutive stages of mass spectrometric fragmentation. Proc Natl Acad Sci USA 101(37):13417–13422
Zhu W, Smith JW, Huang CM (2010) Mass spectrometry-based label-free quantitative proteomics. J Biomed Biotechnol 2010:840518
Yang X, Turke AB, Qi J, Song Y, Rexer BN, Miller TW, Jänne PA, Arteaga CL, Cantley LC, Engelman JA, Asara JM (2011) Using tandem mass spectrometry in targeted mode to identify activators of class IA PI3K in cancer. Cancer Res 71(18):5965–5975
Stuelsatz P, Pouzoulet F, Lamarre Y, Dargelos E, Poussard S, Leibovitch S, Cottin P, Veschambre P (2010) Down-regulation of MyoD by calpain 3 promotes generation of reserve cells in C2C12 myoblasts. J Biol Chem 285(17):12670–12683
Gnocchi VF, White RB, Ono Y, Ellis JA, Zammit PS (2009) Further characterisation of the molecular signature of quiescent and activated mouse muscle satellite cells. PLoS One 4:e5205
Ver Heyen M, Reed TD, Blough RI, Baker DL, Zilberman A, Loukianov E, Van Baelen K, Raeymaekers L, Periasamy M, Wuytack F (2000) Structure and organization of the mouse Atp2a2 gene encoding the sarco(endo)plasmic reticulum Ca2+ -ATPase 2 (SERCA2) isoforms. Mamm Genome 11(2):159–163
Arai M, Otsu K, MacLennan DH, Periasamy M (1992) Regulation of sarcoplasmic reticulum gene expression during cardiac and skeletal muscle development. Am J Physiol Cell Physiol 262((3Pt1)):C614–C620
Dally S, Corvazier E, Bredoux R, Bobe R, Enouf J (2010) Multiple and diverse coexpression, location, and regulation of additional SERCA2 and SERCA3 isoforms in nonfailing and failing human heart. J Mol Cell Cardiol 48(4):633–644
Dremina ES, Sharov VS, Davies MJ, Schöneich C (2007) Oxidation and inactivation of SERCA by selective reaction of cysteine residues with amino acid peroxides. Chem Res Toxicol 20(10):1462–1469
Ho AT, Li QH, Hakem R, Mak TW, Zacksenhaus E (2004) Coupling of caspase-9 to Apaf1 in response to loss of pRb or cytotoxic drugs is cell-type-specific. EMBO J 23(2):460–472
Kamradt MC, Chen F, Sam S, Cryns VL (2002) The small heat shock protein alpha B-crystallin negatively regulates apoptosis during myogenic differentiation by inhibiting caspase-3 activation. J Biol Chem 277(41):38731–38736
Zhang K, Sha J, Harter ML (2010) Activation of Cdc6 by MyoD is associated with the expansion of quiescent myogenic satellite cells. J Cell Biol 188(1):39–48
Tapscott SJ (2005) The circuitry of a master switch: Myod and the regulation of skeletal muscle gene transcription. Development 132(12):2685–2695
Hirai H, Verma M, Watanabe S, Tastad C, Asakura Y, Asakura A (2010) MyoD regulates apoptosis of myoblasts through microRNA-mediated down-regulation of Pax3. J Cell Biol 191(2):347–365
Sabourin LA, Girgis-Gabardo A, Seale P, Asakura A, Rudnicki MA (1999) Reduced differentiation potential of primary MyoD−/− myogenic cells derived from adult skeletal muscle. J Cell Biol 144(4):631–643
Yablonka-Reuveni Z, Rudnicki MA, Rivera AJ, Primig M, Anderson JE, Natanson P (1999) The transition from proliferation to differentiation is delayed in satellite cells from mice lacking MyoD. Dev Biol 210(2):440–455
Asakura A, Hirai H, Kablar B, Morita S, Ishibashi J, Piras BA, Christ AJ, Verma M, Vineretsky KA, Rudnicki MA (2007) Increased survival of muscle stem cells lacking the MyoD gene after transplantation into regenerating skeletal muscle. Proc Natl Acad Sci USA 104(42):16552–16557
Distelhorst CW, Bootman MD (2011) Bcl-2 interaction with the inositol 1,4,5-trisphosphate receptor: role in Ca(2+) signaling and disease. Cell Calcium 50(3):234–241
Acknowledgments
This study was supported by the NIH (P01AG012993). The authors gratefully acknowledge Drs. J. Enouf and R. Bobe (Inserm, Hopital Lariboisiere, Paris, France) for the gift of anti-SERCA2B antibodies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schöneich, C., Dremina, E., Galeva, N. et al. Apoptosis in differentiating C2C12 muscle cells selectively targets Bcl-2-deficient myotubes. Apoptosis 19, 42–57 (2014). https://doi.org/10.1007/s10495-013-0922-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-013-0922-7