Abstract
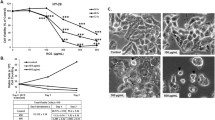
In the present study, we investigate the effect of curcumin, a major active component isolated from rhizomes of Curcuma longa, on the cytotoxicity of three human carcinoma cell lines (AGS, HT-29 and MGC803) in gastrointestinal tract and a normal gastric epithelial cell line GES-1, and the mechanism of curcumin-induced apoptosis. The results indicated that curcumin inhibited the gastrointestinal carcinoma cell growth in a dose-dependent manner and cytotoxicity was more towards the gastric carcinoma cell AGS and colon carcinoma cell HT-29 compared to normal gastric cell GES-1, and increased externalization of phosphatidylserine residue was observed by Annexin V/PI staining in the two cell lines. Treatment of AGS and HT-29 cells with curcumin enhanced the cleavage of procaspase-3, -7, -8 and -9. Meanwhile, curcumin induced endoplasmic reticulum (ER) stress and mitochondrial dysfunction as evidenced by up-regulation of CCAAT/enhancer binding protein homologous protein (CHOP), phosphorylation of JNK and down-regulation of SERCA2ATPase, release of cytochrome c, decrease of Bcl-2 and reduction of mitochondrial membrane potential in both AGS and HT-29 cells. Overexpression of bax, total JNK, phospho-FADD and total FADD were also observed in curcumin-treated HT-29 cells. Moreover, curcumin decreased cytosolic and ER Ca2+, but increased mitochondrial Ca2+ in the two cell lines. 2-Aminoethoxydiphenyl borate, an antagonist of inositol 1, 4, 5-triphosphate receptor, partly blocked curcumin-induced cytosolic Ca2+ decrease in AGS and HT-29 cells. Additionally, carbonyl cyanide m-chlorophenylhydrazone, an inhibitor of mitochondrial Ca2+ uptake, reversed curcumin-triggered AGS and HT-29 cells growth inhibition. siRNA to CHOP markedly reduced curcumin-induced apoptosis. These results suggest that curcumin can impact on ER stress and mitochondria functional pathways in AGS and HT-29 cells, death receptor pathway was also involved in curcumin-treated HT-29 cells, thus identifying specific well-defined molecular mechanisms that may be targeted by therapeutic strategies.








Similar content being viewed by others
Abbreviations
- [Ca2+]c :
-
Cytosolic calcium
- [Ca2+]ER :
-
Endoplasmic reticulum calcium
- [Ca2+]Mito :
-
Mitochondrial calcium
- ER:
-
Endoplasmic reticulum
- CHOP:
-
CCAAT/enhancer binding protein homologous protein
- IP3 :
-
Inositol 1,4,5-triphosphate
- CCCP:
-
Carbonyl cyanide m-chlorophenylhydrazone
- ROS:
-
Reactive oxygen species
- MTT:
-
3-(4,5)-Dimethylthiahiazo(-z-y1)-3,5-diphenytetrazoliumromide
- PI:
-
Propidium iodide
- Fura-2 AM:
-
Fura-2 acetoxymethyl ester
- GRP78/BiP:
-
Glucose-regulated protein 78/Binding immunoglobulin protein
- 2-APB:
-
2-Aminoethoxydiphenyl borate
References
Hengartner MO (2000) The biochemistry of apoptosis. Nature 407(6805):770–776
Grimm S, Brdiczka D (2007) The permeability transition pore in cell death. Apoptosis 12(5):841–855
Gorman AM, Healy SJ, Jager R, Samali A (2012) Stress management at the ER: regulators of ER stress-induced apoptosis. Pharmacol Ther 134(3):306–316. doi:10.1016/j.pharmthera.2012.02.003
Wu Y, Fabritius M, Ip C (2009) Chemotherapeutic sensitization by endoplasmic reticulum stress: increasing the efficacy of taxane against prostate cancer. Cancer Biol Ther 8(2):146–152
Oyadomari S, Mori M (2004) Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11(4):381–389
Luciani DS, Gwiazda KS, Yang TL, Kalynyak TB, Bychkivska Y, Frey MH, Jeffrey KD, Sampaio AV, Underhill TM, Johnson JD (2009) Roles of IP3R and RyR Ca2+ channels in endoplasmic reticulum stress and beta-cell death. Diabetes 58(2):422–432. doi:10.2337/db07-1762
Belakavadi M, Salimath BP (2005) Mechanism of inhibition of ascites tumor growth in mice by curcumin is mediated by NF-kB and caspase activated DNase. Mol Cell Biochem 273(1–2):57–67
Weir NM, Selvendiran K, Kutala VK, Tong L, Vishwanath S, Rajaram M, Tridandapani S, Anant S, Kuppusamy P (2007) Curcumin induces G2/M arrest and apoptosis in cisplatin-resistant human ovarian cancer cells by modulating Akt and p38 MAPK. Cancer Biol Ther 6(2):178–184
Pongrakhananon V, Nimmannit U, Luanpitpong S, Rojanasakul Y, Chanvorachote P (2010) Curcumin sensitizes non-small cell lung cancer cell anoikis through reactive oxygen species-mediated Bcl-2 downregulation. Apoptosis 15(5):574–585. doi:10.1007/s10495-010-0461-4
Javvadi P, Segan AT, Tuttle SW, Koumenis C (2008) The chemopreventive agent curcumin is a potent radiosensitizer of human cervical tumor cells via increased reactive oxygen species production and overactivation of the mitogen-activated protein kinase pathway. Mol Pharmacol 73(5):1491–1501. doi:10.1124/mol.107.043554
Bhaumik S, Anjum R, Rangaraj N, Pardhasaradhi BV, Khar A (1999) Curcumin mediated apoptosis in AK-5 tumor cells involves the production of reactive oxygen intermediates. FEBS Lett 456(2):311–314
Thayyullathil F, Chathoth S, Hago A, Patel M, Galadari S (2008) Rapid reactive oxygen species (ROS) generation induced by curcumin leads to caspase-dependent and -independent apoptosis in L929 cells. Free Radic Biol Med 45(10):1403–1412. doi:10.1016/j.freeradbiomed.2008.08.014
Jaiswal AS, Marlow BP, Gupta N, Narayan S (2002) Beta-catenin-mediated transactivation and cell–cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene 21(55):8414–8427
Jung EM, Lim JH, Lee TJ, Park JW, Choi KS, Kwon TK (2005) Curcumin sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis through reactive oxygen species-mediated upregulation of death receptor 5 (DR5). Carcinogenesis 26(11):1905–1913
Wang M, Ruan Y, Chen Q, Li S, Wang Q, Cai J (2011) Curcumin induced HepG2 cell apoptosis-associated mitochondrial membrane potential and intracellular free Ca2+ concentration. Eur J Pharmacol 650(1):41–47. doi:10.1016/j.ejphar.2010.09.049
Logan-Smith MJ, Lockyer PJ, East JM, Lee AG (2001) Curcumin, a molecule that inhibits the Ca2+-ATPase of sarcoplasmic reticulum but increases the rate of accumulation of Ca2+. J Biol Chem 276(50):46905–46911
Bilmen JG, Khan SZ, Javed MH, Michelangeli F (2001) Inhibition of the SERCA Ca2+ pumps by curcumin. Curcumin putatively stabilizes the interaction between the nucleotide-binding and phosphorylation domains in the absence of ATP. Eur J Biochem 268(23):6318–6327
Sumbilla C, Lewis D, Hammerschmidt T, Inesi G (2002) The slippage of the Ca2+ pump and its control by anions and curcumin in skeletal and cardiac sarcoplasmic reticulum. J Biol Chem 277(16):13900–13906
Yang J, Wu LJ, Tashiro S, Onodera S, Ikejima T (2008) Nitric oxide activated by p38 and NF-kappaB facilitates apoptosis and cell cycle arrest under oxidative stress in evodiamine-treated human melanoma A375–S2 cells. Free Radic Res 42(1):1–11. doi:10.1080/10715760701762407
Ferreiro E, Costa R, Marques S, Cardoso SM, Oliveira CR, Pereira CM (2008) Involvement of mitochondria in endoplasmic reticulum stress-induced apoptotic cell death pathway triggered by the prion peptide PrP(106–126). J Neurochem 104(3):766–776
Fernandez SF, Huang MH, Davidson BA, Knight PR 3rd, Izzo JL Jr (2005) Mechanisms of angiotensin II-mediated decreases in intraneuronal Ca2+ in calcium-loaded stellate ganglion neurons. Hypertension 45(2):276–282
Limke TL, Otero-Montanez JK, Atchison WD (2003) Evidence for interactions between intracellular calcium stores during methylmercury-induced intracellular calcium dysregulation in rat cerebellar granule neurons. J Pharmacol Exp Ther 304(3):949–958
Wu Y, Zhang H, Dong Y, Park YM, Ip C (2005) Endoplasmic reticulum stress signal mediators are targets of selenium action. Cancer Res 65:9073–9079
Misra UK, Pizzo SV (2010) Modulation of the unfolded protein response in prostate cancer cells by antibody-directed against the carboxyl-terminal domain of GRP78. Apoptosis 15(2):173–182. doi:10.1007/s10495-009-0430-y
Strimpakos AS, Sharma RA (2008) Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal 10(3):511–545. doi:10.1089/ars.2007.1769
Eigner D, Scholz D (1999) Ferula asa-foetida and Curcuma longa in traditional medical treatment and diet in Nepal. J Ethnopharmacol 67:1–6
Sharma RA, Euden SA, Platton SL et al (2004) Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res 10:6847–6854
Fang M, Jin Y, Bao W et al (2012) In vitro characterization and in vivo evaluation of nanostructured lipid curcumin carriers for intragastric administration. Int J Nanomedicine 7:5395–5404
Lin HH, Huang HP, Huang CC, Chen JH, Wang CJ (2005) Hibiscus polyphenol-rich extract induces apoptosis in human gastric carcinoma cells via p53 phosphorylation and p38 MAPK/FasL cascade pathway. Mol Carcinog 43:86–99
Oz HS, Ebersole JL (2010) Green tea polyphenols mediated apoptosis in intestinal epithelial cells by a Fadd-Dependent Pathway. J Cancer Ther 1:105–113
Rauert H, Stuhmer T, Bargou R, Wajant H, Siegmund D (2011) TNFR1 and TNFR2 regulate the extrinsic apoptotic pathway in myeloma cells by multiple mechanisms. Cell Death Dis 2:e194
Viswanath V, Wu Y, Boonplueang R, Chen S, Stevenson FF, Yantiri F, Yang L, Beal MF, Andersen JK (2001) Caspase-9 activation results in downstream caspase-8 activation and bid cleavage in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease. J Neurosci 21(24):9519–9528
Li H, Zhu H, Xu CJ, Yuan J (1998) Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94(4):491–501
Zheng HC, Sun JM, Wei ZL, Yang XF, Zhang YC, Xin Y (2003) Expression of Fas ligand and caspase-3 contributes to formation of immune escape in gastric cancer. World J Gastroenterol 9(7):1415–1420
Bennett MW, O’Connell J, O’Sullivan GC, Roche D, Brady C, Kelly J, Collins JK, Shanahan F (1999) Expression of Fas ligand by human gastric adenocarcinomas: a potential mechanism of immune escape in stomach cancer. Gut 44(2):156–162
Bakhshi J, Weinstein L, Poksay KS, Nishinaga B, Bredesen DE, Rao RV (2008) Coupling endoplasmic reticulum stress to the cell death program in mouse melanoma cells: effect of curcumin. Apoptosis 13(7):904–914. doi:10.1007/s10495-008-0221-x
Binet F, Chiasson S, Girard D (2010) Evidence that endoplasmic reticulum (ER) stress and caspase-4 activation occur in human neutrophils. Biochem Biophys Res Commun 391(1):18–23. doi:10.1016/j.bbrc.2009.10.141
Esther AO, Lawrence HB (2005) Caspase-12 and caspase-4 are not required for caspase-dependent endoplasmic reticulum stress-induced apoptosis. J Biol Chem 280:29578–29597
McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ (2001) Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol 21(4):1249–1259
Sanchez AM, Martinez-Botas J, Malagarie-Cazenave S, Olea N, Vara D, Lasuncion MA, Diaz-Laviada I (2008) Induction of the endoplasmic reticulum stress protein GADD153/CHOP by capsaicin in prostate PC-3 cells: a microarray study. Biochem Biophys Res Commun 372(4):785–791. doi:10.1016/j.bbrc.2008.05.138
Liu H, Qian J, Wang F, Sun X, Xu X, Xu W, Zhang X (2010) Expression of two endoplasmic reticulum stress markers, GRP78 and GADD153, in rat retinal detachment model and its implication. Eye (Lond) 24(1):137–144. doi:10.1038/eye.2009.20
Park MT, Song MJ, Lee H, Oh ET, Choi BH, Jeong SY, Choi EK, Park HJ (2011) Beta-lapachone significantly increases the effect of ionizing radiation to cause mitochondrial apoptosis via JNK activation in cancer cells. PLoS ONE 6(10):e25976. doi:10.1371/journal.pone.0025976
Li S, Hao B, Lu Y, Yu P, Lee HC, Yue J (2012) Intracellular alkalinization induces cytosolic Ca2+ increases by inhibiting sarco/endoplasmic reticulum Ca2+-ATPase (SERCA). PLoS ONE 7(2):e31905. doi:10.1371/journal.pone.0031905
Bae JH, Park JW, Kwon TK (2003) Ruthenium red, inhibitor of mitochondrial Ca2+ uniporter, inhibits curcumin-induced apoptosis via the prevention of intracellular Ca2+ depletion and cytochrome c release. Biochem Biophys Res Commun 303(4):1073–1079
Foyouzi-Youssefi R, Arnaudeau S, Borner C, Kelley WL, Tschopp J, Lew DP, Demaurex N, Krause KH (2000) Bcl-2 decreases the free Ca2+ concentration within the endoplasmic reticulum. Proc Natl Acad Sci USA 97(11):5723–5728
Acknowledgments
This study was supported by grants from National Science and Technology Major Project of China (2009ZX09311-003); the Opening Project of Shanghai Key Laboratory of Complex Prescription, Shanghai Science and Technology Committee, P.R. China (Number 10DZ2270900); the Project of Shanghai Leading Academic Discipline, Shanghai Education Committee (J50305), P.R. China; and E-Institute of Traditional Chinese Medicine Internal Medicine, Shanghai Municipal Education Commission (E03008), P.R. China.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Aili Cao and Qi Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Cao, A., Li, Q., Yin, P. et al. Curcumin induces apoptosis in human gastric carcinoma AGS cells and colon carcinoma HT-29 cells through mitochondrial dysfunction and endoplasmic reticulum stress. Apoptosis 18, 1391–1402 (2013). https://doi.org/10.1007/s10495-013-0871-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-013-0871-1