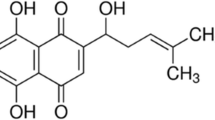
Abstract
Although caspases have been demonstrated to be involved in artemisinin (ARTE)-induced apoptosis, their exact functions are not well understood. The aim of this report is to explore the roles of caspase-8, -9 and -3 during ARTE-induced apoptosis in human lung adenocarcinoma (ASTC-a-1) cells. ARTE treatment induces a rapid generation of reactive oxygen species (ROS), and ROS-dependent apoptosis as well as the activation of caspase-8, -9 and -3 via time- and dose-dependent fashion. Of upmost importance, inhibition of caspase-8 or -9, but not caspase-3, almost completely blocks the ARTE-induced not only activation of the caspase-8, -9 and -3 but also apoptosis. In addition, the apoptotic process triggered by ARTE does not involve the Bid cleavage, tBid translocation, significant loss of mitochondrial membrane potential and cytochrome c release from mitochondria. Moreover, silencing Bax/Bak does not prevent the ATRE-induced cell death as well as the activation of caspase-8, -9 and -3. Collectively, our data firstly demonstrate that ARTE triggers a ROS-mediated positive feedback amplification activation loop between caspase-8 and -9 independent of mitochondria, which dominantly mediated the ARTE-induced apoptosis via a caspase-3-independent apoptotic pathway in ASTC-a-1 cells. Our findings imply a potential to develop new derivatives from artemisinin to effectively initiate the amplification activation loop of caspases.






Similar content being viewed by others
References
Park BK, O’Neill PM, Maggs JL, Pirmohamed M (1998) Safety assessment of peroxide antimalarials: clinical and chemical perspectives. Brit J Clin Pharmaco 46:521–529
Efferth T, Dunstan H, Sauerbrey A, Miyachi H, Chitambar CR (2001) The anti-malarial artesunate is also active against cancer. Int J Oncol 18:767–773
Sunder SN, Marconett CN, Doan VB, Willoughby JA Sr, Firestone GL (2008) Artemisinin selectively decreases functional levels of estrogen receptor–alpha and ablates estrogen-induced proliferation in human breast cancer cells. Carcinogenesis 29:2252–2258
Willoughby JA Sr, Sundar SN, Cheung M, Tin AS, Modiano J, Firestone GL (2009) Artemisinin blocks prostate cancer growth and cell cycle progression by disrupting sp1 interactions with the cyclin-dependent kinase-4 promoter and inhibiting CDK4 gene expression. J Biol Chem 284:2203–2213
Michaelis M, Kleinschmidt MC, Barth S, Rothweiler F, Geiler J, Breitling R, Mayerd B, Deubzere H, Witte O, Kreuterf J, Doerra HW, Cinatl J, Cinatl J Jr (2010) Anti-cancer effects of artesunate in a panel of chemoresistant neuroblastoma cell lines. Biochem Pharmacol 79:130–136
Mercer AE, Maggs JL, Sun XM, Cohen GM, Chadwick J, O’Neill PM, Park K (2007) Evidence for the involvement of carbon-centered radials in the induction of apoptotic cell death by artemisinin compounds. J Biol Chem 282:9372–9382
Mercer AE, Copple IM, Maggs JL, O’Neill PM, Park BK (2011) The role of heme and the mitochondrion in the chemical and molecular mechanisms of mammalian cell death induced by the artemisinin antimalarials. J Biol Chem 286:987–996
Han YH, Kim SZ, Kim SH, Park WH (2008) Induction of apoptosis in arsenic trioxide-treated lung cancer A549 cells by buthionine sulfoximine. Mol Cells 26:158–164
Lu YY, Chen TS, Wang XP, Li L (2010) Single-cell analysis of dihydroartemisinin-induced apoptosis through reactive oxygen species-mediated caspase-8 activation and mitochondrial pathway in ASTC-a-1 cells using fluorescence imaging techniques. J Biomed Opt 15:046028
Lu YY, Chen TS, Wang XP, Qu JL, Chen M (2010) The JNK inhibitor SP600125 enhances dihydroartemisinin-induced apoptosis by accelerating Bax translocation into mitochondria in human lung adenocarcinoma cells. FEBS Lett 584:4019–4026
Earnshaw WC, Martins LM, Kaufmann SH (1999) Mammalian caspases: structure, activation, substrates and functions during apoptosis. Annu Rev Biochem 68:383–424
Gil J, García MA, Esteban M (2002) Caspase 9 activation by the dsRNA-dependent protein kinase, PKR: molecular mechanism and relevance. FEBS Lett 529:249–255
McDonnell MA, Wang D, Khan SM, Heiden MGV, Kelekar A (2003) Caspase-9 is activated in a cytochrome c-independent manner early during TNF-α-induced apoptosis in murine cells. Cell Death Differ 10:1005–1015
McDonnell MA, Abedin MJ, Manuel M, Platikanova TN, Ecklund JR, Ahmed K, Kelekar A (2008) Phosphorylation of murine caspase-9 by the protein kinase casein kinase 2 regulates its cleavage by caspase-8. J Biol Chem 283:20149–20158
Metkar SS, Wang B, Ebbs ML, Kim JH, Lee YJ, Raja SM, Froelich CJ (2003) Granzyme B activates procaspase-3 which signals a mitochondrial amplification loop for maximal apoptosis. J Cell Biol 160:875–885
Chandra D, Choy G, Deng XD, Bhatia B, Daniel P, Tang DG (2004) Association of active caspase 8 with the mitochondrial membrane during apoptosis: potential roles in cleaving BAP31 and caspase 3 and mediating mitochondrion-endoplasmic reticulum cross talk in etoposide-induced cell death. Mol Cell Biol 24:6592–6607
Sitailo LA, Tibudan SS, Denning MF (2002) Activation of caspase-9 is required for UV-induced apoptosis of human keratinocytes. J Biol Chem 277:19346–19352
Li-Weber M (2010) Targeting apoptosis pathways in cancer by Chinese medicine. Cancer Lett. doi:10.1016/j.canlet.2010.07.015
Day TW, Wu CH, Safa AR (2009) Etoposide induces protein kinase Cδ- and caspase-3-dependent apoptosis in neuroblastoma cancer cells. Mol Pharmacol 76:632–640
Minichsdorfer C, Hohennegger M (2009) Autocrine amplification loop in statin-induced apoptosis of human melanoma cells. Br J Pharmacol 157:1278–1290
Wu YX, Xing D, Luo SM, Tang YH, Chen Q (2006) Detection of caspase-3 activation in single cells by fluorescence resonance energy transfer during photodynamic therapy induced apoptosis. Cancer Lett 235:239–247
Lin CF, Chen CL, Chang WT, Jan MS, Hsu LJ, Wu RH, Fang YT, Tang MJ, Chang WC, Lin YS (2005) Bcl-2 rescues ceramide- and etoposide-induced mitochondrial apoptosis through blockage of caspase-2 activation. J Biol Chem 280:23758–23765
Heine K, Pust S, Enzenmüller S, Barth H (2008) ADP-ribosylation of actin by the clostridium botulinum C2 toxin in mammalian cells results in delayed caspase-dependent apoptotic cell death. Infect Immun 76:4600–4608
Jin D, Ojcius DM, Sun D, Dong HY, Luo YH, Mao YF, Yan J (2009) Leptospira interrogans induces apoptosis in macrophages via caspase-8- and caspase-3-dependent pathways. Infect Immun 77:799–809
Kim H, Tu HC, Ren D, Takeuchi O, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH (2009) Stepwise activation of Bax and Bak by tBid, Bim, and Puma initiates mitochondrial apoptosis. Mol Cell 36:487–499
Li HL, Zhu H, Xu CJ, Yuan JY (1998) Cleavage of Bid by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491–501
Thornberry NA, Lazebnik Y (1998) Caspases: enemies within. Science 281:1312–1316
Lovell JF, Billen LP, Bindner S, Din AS, Fradin C, Leber B, Andrews DW (2008) Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell 135:1074–1084
Wu YY, Xing D, Chen WR, Wang XC (2007) Bid is not required for Bax translocation during UV-induced apoptosis. Cell Signal 19:2468–2478
Madesh M, Antonsson B, Srinivasula SM, Alnemri ES, Hajnóczky G (2002) Rapid kinetics of tBid-induced cytochrome c and Smac/DIABLO release and mitochondrial depolarization. J Biol Chem 277:5651–5659
Hansen MG, Farkas T, Fehrenbacher N et al (2006) Apoptosome-independent activation of the lysosomal cell death pathway by caspase-9. Mol Cell Biol 26:7880–7891
Wang XD (2001) The expanding role of mitochondria in apoptosis. Gene Dev 15:2922–2933
Tsujimoto Y (2003) Cell death regulation by the Bcl-2 protein family in the mitochondria. J Cell Physiol 195:158–167
Wasiak S, Zunino R, McBride HM (2007) Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J Cell Biol 177:439–450
Efferth T, Briehl MM, Tome ME (2003) Role of antioxidant genes for the activity of artesunate against tumor cells. Int J Oncol 23:1231–1235
Efferth T, Benakis A, Romero MR et al (2004) Enhancement of cytotoxicity of artemisinins toward cancer cells by ferrous iron. Free Radical Bio Med 37:998–1009
Shukla S, Gupta S (2008) Apigenin-induced prostate cancer cell death is initiated by reactive oxygen species and p53 activation. Free Radical Bio Med 44:1833–1845
Benhar M, Engelberg D, Levitzki A (2002) ROS, stress-activated kinases and stress signaling in cancer. EMBO Rep 3:420–425
Buccellato LJ, Tso M, Akinci OI, Chandel NS, Budinger GR (2004) Reactive oxygen species are required for hyperoxia-induced Bax activation and cell death in alveolar epithelial cells. J Biol Chem 279:6753–6760
Efferth T, Rücker G, Falkenberg M, Manns D, Olbrich A, Fabry U, Osieka R (1996) Detection of apoptosis in KG-1a leukemic cells treated with investigational drugs. Arzneimittelforschung 46:196–200
Efferth T, Giaisi M, Merling A, Krammer PH, Li-Weber M (2007) Artesunate induces ROS-mediated apoptosis in doxorubicin-resistant T leukemia cells. PLoS ONE 2(8):e693. doi:10.1371/journal.pone.0000693
Hamacher-Brady A, Stein HA, Turschner S, Toegel I, Mora R, Jennewein N, Efferth T, Eils R, Brady NR (2011) Artesunate activates mitochondrial apoptosis in breast cancer cells via iron-catalyzed lysosomal reactive oxygen species production. J Biol Chem 286:6587–6601
Chen T, Li M, Zhang RW, Wang H (2009) Dihydroartemisinin induces apoptosis and sensitizes human ovarian cancer cells to carboplatin therapy. J Cell Mol Med 13:1358–1370
Handrick R, Ontikatze T, Bauer KD, Freier F, Rübel A, Dürig J Belka C, Jendrossek V (2010) Dihydroartemisinin induces apoptosis by a Bak-dependent intrinsic pathway. Mol Cancer Ther 9:2497–2510
Beekman AC, Woerdenbag HJ, Van UW, Pras N, Konings AW, Wikström HV (1997) Stability of artemisinin in aqueous environments: impact on its cytotoxic action to ehrlich ascites tumour cells. J Pharm Pharmacol 49:1254–1258
Beekman AC, Wierenga PK, Woerdenbag HJ et al (1998) Artemisinin-derived sesquiterpene lactones as potential antitumour compounds: cytotoxic action against bone marrow and tumour cells. Planta Med 64:615–619
Circu ML, Aw TY (2010) Reactive oxygen species, cellular redox systems, and apoptosis. Free Radical Bio Med 48:749–762
Huang DCS, Hahne M, Schroeter M et al (1999) Activation of Fas by FasL induces apoptosis by a mechanism that cannot be blocked by Bcl-2 or Bcl-xL. P Natl Acad Sci USA 96:14871–14876
Hsieh MH, Korngold R (2000) Differential use of FasL- and perforin-mediated cytolytic mechanisms by T-cell subsets involved in graft-versus-myeloid leukemia responses. Blood 96:1047–1055
Acknowledgment
We thank Dr. K. Taira for providing CFP-Bid plasmid and Dr. Y. Gotoh for providing DsRed-Mito plasmid. This work was supported by National Natural Science Foundation of China (31071218, 61178078 and 81071491) and Key Project of the Department of Education and Finance of Guangdong Province (cxzd115).
Author information
Authors and Affiliations
Corresponding author
Additional information
Fenglian Xiao and Weijie Gao contribute equally to this paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xiao, F., Gao, W., Wang, X. et al. Amplification activation loop between caspase-8 and -9 dominates artemisinin-induced apoptosis of ASTC-a-1 cells. Apoptosis 17, 600–611 (2012). https://doi.org/10.1007/s10495-012-0706-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-012-0706-5