Abstract
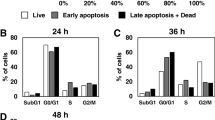
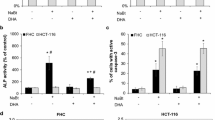
Previous studies suggest that the n-3 polyunsaturated fatty acids (PUFAs) eicosapenteinoic acid (EPA) and docosahexaenoic acid (DHA), constituents of fish oil, exert chemopreventive activity in colon cancer. One of the mechanisms involved is the facilitation of apoptosis. While a pro-apoptotic potential of n-3 PUFAs has been suggested, it is still unclear whether additional consumption of fish will also lead to comparable results. The aim of this study was to assess EPA- and DHA-mediated effects on endpoints of apoptosis and to use a novel biomarker-approach to measure modulation of apoptosis by consumption of fish. LT97 human colon adenoma and HT29 human colon adenocarcinoma cells were used to investigate modulation of apoptosis by EPA, DHA or linoleic acid (LA) using a set of endpoints, namely phosphatidylserine staining with Annexin-V (flow cytometry), Bcl-2 expression (Real-time RT–PCR), and Bid, caspase 3, 8 and 9 expression as well as PARP cleavage (Western Blot). Furthermore, faecal water (FW) of volunteers (n = 89) from a human trial intervening with fish was used to investigate changes in apoptosis by flow cytometry. DHA was more effective at inducing apoptosis than EPA. LT97 cells were more prone to DHA and EPA induced apoptosis than HT29 cells. Treatment of LT97 cells with FW from volunteers consuming fish did not result in any changes in apoptosis. Taken together, our results show that adenoma cells are highly susceptible to n-3 PUFA-induced apoptosis. By using a biomarker-approach (FW) to measure apoptosis-induction ex vivo no change in apoptosis after additional fish consumption was detectable.







Similar content being viewed by others
References
Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P (2007) Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol 18:581–592
Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ (2006) Cancer statistics, 2006. CA Cancer J Clin 56:106–130
Fearon ER, Vogelstein B (1990) A genetic model for colorectal tumorigenesis. Cell 61:759–767
Martinez ME, Marshall JR, Giovannucci E (2008) Diet and cancer prevention: the roles of observation and experimentation. Nat Rev Cancer 8:694–703
Sporn MB (1991) Carcinogenesis and cancer: different perspectives on the same disease. Cancer Res 51:6215–6218
Cheng J, Ogawa K, Kuriki K, Yokoyama Y, Kamiya T, Seno K, Okuyama H, Wang J, Luo C, Fujii T, Ichikawa H, Shirai T, Tokudome S (2003) Increased intake of n-3 polyunsaturated fatty acids elevates the level of apoptosis in the normal sigmoid colon of patients polypectomized for adenomas/tumours. Cancer Lett 193:17–24
Courtney ED, Matthews S, Finlayson C, Di PD, Belluzzi A, Roda E, Kang JY, Leicester RJ (2007) Eicosapentaenoic acid (EPA) reduces crypt cell proliferation and increases apoptosis in normal colonic mucosa in subjects with a history of colorectal adenomas. Int J Colorectal Dis 22:765–776
Dommels YE, Heemskerk S, Van Den-Berg H, Alink GM, Van Bladeren PJ, Van Ommen B (2003) Effects of high fat fish oil and high fat corn oil diets on initiation of AOM-induced colonic aberrant crypt foci in male F344 rats. Food Chem Toxicol 41:1739–1747
Latham P, Lund EK, Johnson IT (1999) Dietary n-3 PUFA increases the apoptotic response to 1, 2-dimethylhydrazine, reduces mitosis and suppresses the induction of carcinogenesis in the rat colon. Carcinogenesis 20:645–650
Reddy BS, Sugie S (1988) Effect of different levels of omega-3 and omega-6 fatty acids on azoxymethane-induced colon carcinogenesis in F344 rats. Cancer Res 48:6642–6647
Geelen A, Schouten JM, Kamphuis C, Stam BE, Burema J, Renkema JM, Bakker EJ, Van Veer P, Kampman E (2007) Fish consumption, n-3 fatty acids, and colorectal cancer: a meta-analysis of prospective cohort studies. Am J Epidemiol 166:1116–1125
Mantzioris E, James MJ, Gibson RA, Cleland LG (1994) Dietary substitution with an alpha-linolenic acid-rich vegetable oil increases eicosapentaenoic acid concentrations in tissues. Am J Clin Nutr 59:1304–1309
Pawlosky RJ, Hibbeln JR, Novotny JA, Salem N Jr (2001) Physiological compartmental analysis of alpha-linolenic acid metabolism in adult humans. J Lipid Res 42:1257–1265
Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A (2004) Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr 79:935–945
Serini S, Piccioni E, Merendino N, Calviello G (2009) Dietary polyunsaturated fatty acids as inducers of apoptosis: implications for cancer. Apoptosis 14:135–152
Pot GK, Majsak-Newman G, Geelen A, Harvey LJ, Nagengast FM, Witteman BJ, van de Meeberg PC, Timmer R, Tan A, Wahab PJ, Hart AR, Williams MP, Przybylska-Phillips K, Dainty JR, Schaafsma G, Kampman E, Lund EK (2009) Fish consumption and markers of colorectal cancer risk: a multicenter randomized controlled trial. Am J Clin Nutr
Klinder A, Karlsson PC, Clune Y, Hughes R, Glei M, Rafter JJ, Rowland I, Collins JK, Pool-Zobel BL (2007) Faecal water as a non-invasive biomarker in nutritional intervention: comparison of preparation methods and refinement of different endpoints. Nutr Cancer 57:158–167
Richter M, Jurek D, Wrba F, Kaserer K, Wurzer G, Karner-Hanusch J, Marian B (2002) Cells obtained from colorectal microadenomas mirror early premalignant growth patterns in vitro. Eur J Cancer 38:1937–1945
Klenow S, Pool-Zobel BL, Glei M (2009) Influence of inorganic and organic iron compounds on parameters of cell growth and survival in human colon cells. Toxicol In Vitro 23:400–407
Knoll N, Weise A, Claussen U, Sendt W, Marian B, Glei M, Pool-Zobel BL (2006) 2-Dodecylcyclobutanone, a radiolytic product of palmitic acid, is genotoxic in primary human colon cells and in cells from preneoplastic lesions. Mutat Res 594:10–19
Fogh J, Trempe X (1975) Human tumour cells in vitro. 115–159
Kawai K, Viars C, Arden K, Tarin D, Urquidi V, Goodison S (2002) Comprehensive karyotyping of the HT-29 colon adenocarcinoma cell line. Genes Chromosomes Cancer 34:1–8
Pot GK, Habermann N, Majsak-Newman G, Harvey LJ, Geelen A, Przybylska-Philips K, Nagengast FM, Witteman BJ, van de Meeberg PC, Hart AR, Schaafsma G, Hooiveld G, Glei M, Lund EK, Pool-Zobel BL, Kampman E (2009) Increasing fish consumption does not affect genotoxicity markers in the colon in an intervention study. Carcinogenesis
Veeriah S, Kautenburger T, Habermann N, Sauer J, Dietrich H, Will F, Pool-Zobel BL (2006) Apple flavonoids inhibit growth of HT29 human colon cancer cells and modulate expression of genes involved in the biotransformation of xenobiotics. Mol Carcinog 45:164–174
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Collett ED, Davidson LA, Fan YY, Lupton JR, Chapkin RS (2001) n-6 and n-3 polyunsaturated fatty acids differentially modulate oncogenic Ras activation in colonocytes. Am J Physiol Cell Physiol 280:C1066–C1075
Denys A, Hichami A, Khan NA (2001) Eicosapentaenoic acid and docosahexaenoic acid modulate MAP kinase (ERK1/ERK2) signaling in human T cells. J Lipid Res 42:2015–2020
Engelbrecht AM, Toit-Kohn JL, Ellis B, Thomas M, Nell T, Smith R (2008) Differential induction of apoptosis and inhibition of the PI3-kinase pathway by saturated, monounsaturated and polyunsaturated fatty acids in a colon cancer cell model. Apoptosis 13:1368–1377
Holian O, Nelson R (1992) Action of long-chain fatty acids on protein kinase C activity: comparison of omega-6 and omega-3 fatty acids. Anticancer Res 12:975–980
Toit-Kohn JL, Louw L, Engelbrecht AM (2009) Docosahexaenoic acid induces apoptosis in colorectal carcinoma cells by modulating the PI3 kinase and p38 MAPK pathways. J Nutr Biochem 20:106–114
Busstra MC, Siezen CL, Grubben MJ, van Kranen HJ, Nagengast FM, Van’t VP (2003) Tissue levels of fish fatty acids and risk of colorectal adenomas: a case-control study (Netherlands). Cancer Causes Control 14:269–276
Pot GK, Geelen A, van Heijningen EM, Siezen CL, van Kranen HJ, Kampman E (2008) Opposing associations of serum n-3 and n-6 polyunsaturated fatty acids with colorectal adenoma risk: an endoscopy-based case-control study. Int J Cancer 123:1974–1977
Latham P, Lund EK, Brown JC, Johnson IT (2001) Effects of cellular redox balance on induction of apoptosis by eicosapentaenoic acid in HT29 colorectal adenocarcinoma cells and rat colon in vivo. Gut 49:97–105
Habermann N, Christian B, Luckas B, Pool-Zobel BL, Lund EK, Glei M (2009) Effects of fatty acids on metabolism and cell growth of human colon cell lines of different transformation state. Biofactors 35:460–467
Chen ZY, Istfan NW (2000) Docosahexaenoic acid is a potent inducer of apoptosis in HT-29 colon cancer cells. Prostaglandins Leukot Essent Fatty Acids 63:301–308
Clarke RG, Lund EK, Latham P, Pinder AC, Johnson IT (1999) Effect of eicosapentaenoic acid on the proliferation and incidence of apoptosis in the colorectal cell line HT29. Lipids 34:1287–1295
Hossain Z, Hosokawa M, Takahashi K (2009) Growth inhibition and induction of apoptosis of colon cancer cell lines by applying marine phospholipid. Nutr Cancer 61:123–130
Yi X, Yin XM, Dong Z (2003) Inhibition of Bid-induced apoptosis by Bcl-2. tBid insertion, Bax translocation, and Bax/Bak oligomerization suppressed. J Biol Chem 278:16992–16999
Vaculova A, Hofmanova J, Andera L, Kozubik A (2005) TRAIL and docosahexaenoic acid cooperate to induce HT-29 colon cancer cell death. Cancer Lett 229:43–48
Simon HU, Haj-Yehia A, Levi-Schaffer F (2000) Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 5:415–418
Perez-Cruz I, Carcamo JM, Golde DW (2003) Vitamin C inhibits FAS-induced apoptosis in monocytes and U937 cells. Blood 102:336–343
Perez-Cruz I, Carcamo JM, Golde DW (2007) Caspase-8 dependent TRAIL-induced apoptosis in cancer cell lines is inhibited by vitamin C and catalase. Apoptosis 12:225–234
Carey MC, Small DM, Bliss CM (1983) Lipid digestion and absorption. Annu Rev Physiol 45:651–677
Saunders DR, Sillery JK (1988) Absorption of triglyceride by human small intestine: dose-response relationships. Am J Clin Nutr 48:988–991
Gee JM, Watson M, Matthew JA, Rhodes M, Speakman CJ, Stebbings WS, Johnson IT (1999) Consumption of fish oil leads to prompt incorporation of eicosapentaenoic acid into colonic mucosa of patients prior to surgery for colorectal cancer, but has no detectable effect on epithelial cytokinetics. J Nutr 129:1862–1865
Hillier K, Jewell R, Dorrell L, Smith CL (1991) Incorporation of fatty acids from fish oil and olive oil into colonic mucosal lipids and effects upon eicosanoid synthesis in inflammatory bowel disease. Gut 32:1151–1155
Marangoni AG (1993) Effects of the interaction of porcine pancreatic lipase with AOT/isooctane reverse micelles on enzyme structure and function follow predictable patterns. Enzyme Microb Technol 15:944–949
Glei M, Habermann N, Osswald K, Seidel C, Persin C, Jahreis G, Pool-Zobel BL (2005) Assessment of DNA damage and its modulation by dietary and genetic factors in smokers using the Comet assay: a biomarker model. Biomarkers 10:203–217
Le Leu RK, Brown IL, Hu Y, Bird AR, Jackson M, Esterman A, Young GP (2005) A symbiotic combination of resistant starch and Bifidobacterium lactis facilitates apoptotic deletion of carcinogen-damaged cells in rat colon. J Nutr 135:996–1001
Acknowledgments
We are very grateful to all the people who kindly participated in the FISHGASTRO study. We thank the FISHGASTRO Study Group, particularly Gerda K. Pot (Division of Human Nutrition, Wageningen University, The Netherlands), Gosia Majsak-Newman and Dr. Linda J. Harvey (Institute of Food Research, Norwich, UK) for organising and carrying out the FISHGASTRO human study. Sylvia Thiele (Department of Nutritional Toxicology, Institute for Nutrition, Friedrich-Schiller-University Jena, Germany) is acknowledged for technical assistance. This work was performed within the Integrated Research Project SEAFOODplus, contract No FOOD-CT-2004-506359. The financing of the work by the European Union is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Habermann, N., Schön, A., Lund, E.K. et al. Fish fatty acids alter markers of apoptosis in colorectal adenoma and adenocarcinoma cell lines but fish consumption has no impact on apoptosis-induction ex vivo. Apoptosis 15, 621–630 (2010). https://doi.org/10.1007/s10495-010-0459-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-010-0459-y