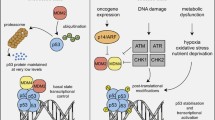
Abstract
The molecular subversion of cell death is acknowledged as a principal contributor to the development and progression of cancer. The p53 tumor suppressor protein is among the most commonly altered proteins in human cancer. The p53 protein mediates critical functions within cells including the response to genotoxic stress, differentiation, senescence, and cell death. Loss of p53 function can result in enhanced rates of cell proliferation, resistance to cell death stimuli, genomic instability, and metastasis. The community of cancer scientists is now in possession of a vast repository of information regarding the frequency, specific mechanisms, and clinical context of cell death deregulation in cancer. This information has enabled the design of therapeutic agents to target proteins, including p53. The feasibility and impact of targeting cell death signaling proteins has been established in preclinical models of human cancer. The appropriate application of these targeted agents is now being established in clinical trials.

Similar content being viewed by others
References
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70. doi:10.1016/S0092-8674(00)81683-9
Igney FH, Krammer PH (2002) Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer 2:277–288. doi:10.1038/nrc776
Evan GI, Vousden KH (2001) Proliferation, cell cycle and apoptosis in cancer. Nature 411:342–348. doi:10.1038/35077213
Chang C, Simmons DT, Martin MA, Mora PT (1979) Identification and partial characterization of new antigens from simian virus 40-transformed mouse cells. J Virol 31:463–471
Kress M, May E, Cassingena R, May P (1979) Simian virus 40-transformed cells express new species of proteins precipitable by anti-simian virus 40 tumor serum. J Virol 31:472–483
Lane DP, Crawford LV (1979) T antigen is bound to a host protein in SV40-transformed cells. Nature 278:261–263. doi:10.1038/278261a0
Linzer DI, Levine AJ (1979) Characterization of a 54 K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell 17:43–52. doi:10.1016/0092-8674(79)90293-9
Melero JA, Stitt DT, Mangel WF, Carroll RB (1979) Identification of new polypeptide species (48–55 K) immunoprecipitable by antiserum to purified large T antigen and present in SV40-infected and -transformed cells. Virology 93:466–480. doi:10.1016/0042-6822(79)90250-2
Eliyahu D, Raz A, Gruss P, Givol D, Oren M (1984) Participation of p53 cellular tumour antigen in transformation of normal embryonic cells. Nature 312:646–649. doi:10.1038/312646a0
Parada LF, Land H, Weinberg RA, Wolf D, Rotter V (1984) Cooperation between gene encoding p53 tumour antigen and ras in cellular transformation. Nature 312:649–651. doi:10.1038/312649a0
Hinds P, Finlay C, Levine AJ (1989) Mutation is required to activate the p53 gene for cooperation with the ras oncogene and transformation. J Virol 63:739–746
Finlay CA, Hinds PW, Levine AJ (1989) The p53 proto-oncogene can act as a suppressor of transformation. Cell 57:1083–1093. doi:10.1016/0092-8674(89)90045-7
Baker SJ, Fearon ER et al (1989) Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science 244:217–221. doi:10.1126/science.2649981
Hollstein M, Rice K et al (1994) Database of p53 gene somatic mutations in human tumors and cell lines. Nucleic Acids Res 22:3551–3555
Wiman KG (2006) Strategies for therapeutic targeting of the p53 pathway in cancer. Cell Death Differ 13:921–926. doi:10.1038/sj.cdd.4401921
Momand J, Jung D, Wilczynski S, Niland J (1998) The MDM2 gene amplification database. Nucleic Acids Res 26:3453–3459. doi:10.1093/nar/26.15.3453
Bond GL, Hu W et al (2004) A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell 119:591–602. doi:10.1016/j.cell.2004.11.022
Kupper M, Joos S et al (2001) MDM2 gene amplification and lack of p53 point mutations in Hodgkin and Reed-Sternberg cells: results from single-cell polymerase chain reaction and molecular cytogenetic studies. Br J Haematol 112:768–775. doi:10.1046/j.1365-2141.2001.02566.x
Hernandez L, Bea S et al (2005) CDK4 and MDM2 gene alterations mainly occur in highly proliferative and aggressive mantle cell lymphomas with wild-type INK4a/ARF locus. Cancer Res 65:2199–2206. doi:10.1158/0008-5472.CAN-04-1526
Marin MC, Jost CA et al (2000) A common polymorphism acts as an intragenic modifier of mutant p53 behaviour. Nat Genet 25:47–54. doi:10.1038/75586
Arva NC, Gopen TR et al (2005) A chromatin-associated and transcriptionally inactive p53-Mdm2 complex occurs in mdm2 SNP309 homozygous cells. J Biol Chem 280:26776–26787. doi:10.1074/jbc.M505203200
Gryshchenko I, Hofbauer S et al (2008) MDM2 SNP309 is associated with poor outcome in B-cell chronic lymphocytic leukemia. J Clin Oncol 26:2252–2257. doi:10.1200/JCO.2007.11.5212
Navone NM, Labate ME et al (1999) p53 mutations in prostate cancer bone metastases suggest that selected p53 mutants in the primary site define foci with metastatic potential. J Urol 161:304–308. doi:10.1016/S0022-5347(01)62136-0
Fearon ER, Vogelstein B (1990) A genetic model for colorectal tumorigenesis. Cell 61:759–767. doi:10.1016/0092-8674(90)90186-I
Benjamin CL, Ananthaswamy HN (2007) p53 and the pathogenesis of skin cancer. Toxicol Appl Pharmacol 224:241–248. doi:10.1016/j.taap.2006.12.006
Purdie CA, Harrison DJ et al (1994) Tumour incidence, spectrum and ploidy in mice with a large deletion in the p53 gene. Oncogene 9:603–609
Donehower LA, Harvey M et al (1992) Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356:215–221. doi:10.1038/356215a0
Jacks T, Remington L et al (1994) Tumor spectrum analysis in p53-mutant mice. Curr Biol 4:1–7. doi:10.1016/S0960-9822(00)00002-6
Clarke AR, Purdie CA et al (1993) Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature 362:849–852. doi:10.1038/362849a0
Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T (1993) p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature 362:847–849. doi:10.1038/362847a0
Schmitt CA, Fridman JS et al (2002) Dissecting p53 tumor suppressor functions in vivo. Cancer Cell 1:289–298. doi:10.1016/S1535-6108(02)00047-8
Roger L, Gadea G, Roux P (2006) Control of cell migration: a tumour suppressor function for p53? Biol Cell 98:141–152. doi:10.1042/BC20050058
Teodoro JG, Evans SK, Green MR (2007) Inhibition of tumor angiogenesis by p53: a new role for the guardian of the genome. J Mol Med 85:1175–1186. doi:10.1007/s00109-007-0221-2
Jin S (2005) p53, Autophagy and tumor suppression. Autophagy 1:171–173
Olive KP, Tuveson DA et al (2004) Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell 119:847–860. doi:10.1016/j.cell.2004.11.004
Lang GA, Iwakuma T et al (2004) Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell 119:861–872. doi:10.1016/j.cell.2004.11.006
Ang HC, Joerger AC, Mayer S, Fersht AR (2006) Effects of common cancer mutations on stability and DNA binding of full-length p53 compared with isolated core domains. J Biol Chem 281:21934–21941. doi:10.1074/jbc.M604209200
Iwakuma T, Lozano G (2007) Crippling p53 activities via knock-in mutations in mouse models. Oncogene 26:2177–2184. doi:10.1038/sj.onc.1210278
Liu G, Parant JM et al (2004) Chromosome stability, in the absence of apoptosis, is critical for suppression of tumorigenesis in Trp53 mutant mice. Nat Genet 36:63–68. doi:10.1038/ng1282
Lozano G, Zambetti GP (2005) What have animal models taught us about the p53 pathway? J Pathol 205:206–220. doi:10.1002/path.1704
Miyashita T, Reed JC (1995) Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80:293–299. doi:10.1016/0092-8674(95)90513-8
Oda E, Ohki R et al (2000) Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288:1053–1058. doi:10.1126/science.288.5468.1053
Nakano K, Vousden KH (2001) PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell 7:683–694. doi:10.1016/S1097-2765(01)00214-3
Xue W, Zender L et al (2007) Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445:656–660. doi:10.1038/nature05529
Ventura A, Kirsch DG et al (2007) Restoration of p53 function leads to tumour regression in vivo. Nature 445:661–665. doi:10.1038/nature05541
Zhang Y, Xiong Y, Yarbrough WG (1998) ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell 92:725–734. doi:10.1016/S0092-8674(00)81401-4
Xu Y, Baltimore D (1996) Dual roles of ATM in the cellular response to radiation and in cell growth control. Genes Dev 10:2401–2410. doi:10.1101/gad.10.19.2401
Hawley RS, Friend SH (1996) Strange bedfellows in even stranger places: the role of ATM in meiotic cells, lymphocytes, tumors, and its functional links to p53. Genes Dev 10:2383–2388. doi:10.1101/gad.10.19.2383
Sherr CJ (1998) Tumor surveillance via the ARF-p53 pathway. Genes Dev 12:2984–2991. doi:10.1101/gad.12.19.2984
Christophorou MA, Ringshausen I, Finch AJ, Swigart LB, Evan GI (2006) The pathological response to DNA damage does not contribute to p53-mediated tumour suppression. Nature 443:214–217. doi:10.1038/nature05077
Efeyan A, Garcia-Cao I, Herranz D, Velasco-Miguel S, Serrano M (2006) Tumour biology: policing of oncogene activity by p53. Nature 443:159. doi:10.1038/443159a
Barak Y, Juven T, Haffner R, Oren M (1993) mdm2 expression is induced by wild type p53 activity. EMBO J 12:461–468
Wu X, Bayle JH, Olson D, Levine AJ (1993) The p53-mdm-2 autoregulatory feedback loop. Genes Dev 7:1126–1132. doi:10.1101/gad.7.7a.1126
Harris SL, Levine AJ (2005) The p53 pathway: positive and negative feedback loops. Oncogene 24:2899–2908. doi:10.1038/sj.onc.1208615
Vousden KH, Lu X (2002) Live or let die: the cell’s response to p53. Nat Rev Cancer 2:594–604. doi:10.1038/nrc864
Wei CL, Wu Q et al (2006) A global map of p53 transcription-factor binding sites in the human genome. Cell 124:207–219. doi:10.1016/j.cell.2005.10.043
Spurgers KB, Coombes KR et al (2004) A comprehensive assessment of p53-responsive genes following adenoviral-p53 gene transfer in Bcl-2-expressing prostate cancer cells. Oncogene 23:1712–1723. doi:10.1038/sj.onc.1207293
Wang L, Wu Q et al (2001) Analyses of p53 target genes in the human genome by bioinformatic and microarray approaches. J Biol Chem 276:43604–43610. doi:10.1074/jbc.M106570200
Riley T, Sontag E, Chen P, Levine A (2008) Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol 9:402–412. doi:10.1038/nrm2395
Robles AI, Bemmels NA, Foraker AB, Harris CC (2001) APAF-1 is a transcriptional target of p53 in DNA damage-induced apoptosis. Cancer Res 61:6660–6664
Chipuk JE, Green DR (2006) Dissecting p53-dependent apoptosis. Cell Death Differ 13:994–1002. doi:10.1038/sj.cdd.4401908
Li Y, Raffo AJ et al (2003) Fas-mediated apoptosis is dependent on wild-type p53 status in human cancer cells expressing a temperature-sensitive p53 mutant alanine-143. Cancer Res 63:1527–1533
Liu T, Laurell C et al (2007) Hypoxia induces p53-dependent transactivation and Fas/CD95-dependent apoptosis. Cell Death Differ 14:411–421. doi:10.1038/sj.cdd.4402022
Danial NN, Korsmeyer SJ (2004) Cell death: critical control points. Cell 116:205–219. doi:10.1016/S0092-8674(04)00046-7
Murphy M, Ahn J et al (1999) Transcriptional repression by wild-type p53 utilizes histone deacetylases, mediated by interaction with mSin3a. Genes Dev 13:2490–2501. doi:10.1101/gad.13.19.2490
Jackson MW, Agarwal MK et al (2005) p130/p107/p105Rb-dependent transcriptional repression during DNA-damage-induced cell-cycle exit at G2. J Cell Sci 118:1821–1832. doi:10.1242/jcs.02307
Sage J, Mulligan GJ et al (2000) Targeted disruption of the three Rb-related genes leads to loss of G(1) control and immortalization. Genes Dev 14:3037–3050. doi:10.1101/gad.843200
Polager S, Ginsberg D (2003) E2F mediates sustained G2 arrest and down-regulation of Stathmin and AIM-1 expression in response to genotoxic stress. J Biol Chem 278:1443–1449. doi:10.1074/jbc.M210327200
Spurgers KB, Gold DL et al (2006) Identification of cell cycle regulatory genes as principal targets of p53-mediated transcriptional repression. J Biol Chem 281:25134–25142. doi:10.1074/jbc.M513901200
Hammond EM, Mandell DJ et al (2006) Genome-wide analysis of p53 under hypoxic conditions. Mol Cell Biol 26:3492–3504. doi:10.1128/MCB.26.9.3492-3504.2006
Wolff S, Erster S, Palacios G, Moll UM (2008) p53’s mitochondrial translocation and MOMP action is independent of Puma and Bax and severely disrupts mitochondrial membrane integrity. Cell Res 18:733–744. doi:10.1038/cr.2008.62
Symonds H, Krall L et al (1994) p53-dependent apoptosis suppresses tumor growth and progression in vivo. Cell 78:703–711. doi:10.1016/0092-8674(94)90534-7
Marchenko ND, Moll UM (2007) The role of ubiquitination in the direct mitochondrial death program of p53. Cell Cycle 6:1718–1723
Ikawa S, Nakagawara A, Ikawa Y (1999) p53 family genes: structural comparison, expression and mutation. Cell Death Differ 6:1154–1161. doi:10.1038/sj.cdd.4400631
Oswald C, Stiewe T (2008) In good times and bad: p73 in cancer. Cell Cycle 7:1726–1731
Rosenbluth JM, Pietenpol JA (2008) The jury is in: p73 is a tumor suppressor after all. Genes Dev 22:2591–2595. doi:10.1101/gad.1727408
Finlan LE, Hupp TR (2007) p63: the phantom of the tumor suppressor. Cell Cycle 6:1062–1071
Flores ER, Tsai KY et al (2002) p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature 416:560–564. doi:10.1038/416560a
Irwin MS (2006) DeltaNp73: misunderstood protein? Cancer Biol Ther 5:804–807
Yang A, Kaghad M et al (1998) p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell 2:305–316. doi:10.1016/S1097-2765(00)80275-0
Yang A, Walker N et al (2000) p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature 404:99–103. doi:10.1038/35003607
Pozniak CD, Radinovic S et al (2000) An anti-apoptotic role for the p53 family member, p73, during developmental neuron death. Science 289:304–306. doi:10.1126/science.289.5477.304
Mills AA, Zheng B et al (1999) p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 398:708–713. doi:10.1038/19531
Yang A, Schweitzer R et al (1999) p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 398:714–718. doi:10.1038/19539
Chang TC, Wentzel EA et al (2007) Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell 26:745–752. doi:10.1016/j.molcel.2007.05.010
Jovanovic M, Hengartner MO (2006) miRNAs and apoptosis: RNAs to die for. Oncogene 25:6176–6187. doi:10.1038/sj.onc.1209912
Cheng AM, Byrom MW, Shelton J, Ford LP (2005) Antisense inhibition of human miRNAs and indications for an involvement of miRNA in cell growth and apoptosis. Nucleic Acids Res 33:1290–1297. doi:10.1093/nar/gki200
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297. doi:10.1016/S0092-8674(04)00045-5
Calin GA, Liu CG et al (2004) MicroRNA profiling reveals distinct signatures in B cell chronic lymphocytic leukemias. Proc Natl Acad Sci USA 101:11755–11760. doi:10.1073/pnas.0404432101
Pichiorri F, Suh SS et al (2008) MicroRNAs regulate critical genes associated with multiple myeloma pathogenesis. Proc Natl Acad Sci USA 105:12885–12890. doi:10.1073/pnas.0806202105
He L, He X et al (2007) A microRNA component of the p53 tumour suppressor network. Nature 447:1130–1134. doi:10.1038/nature05939
Shi XB, Tepper CG, deVere White RW (2008) Cancerous miRNAs and their regulation. Cell Cycle 7:1529–1538
Bottoni A, Piccin D et al (2005) miR-15a and miR-16–1 down-regulation in pituitary adenomas. J Cell Physiol 204:280–285. doi:10.1002/jcp.20282
Croce CM (2008) MicroRNAs and lymphomas. Ann Oncol 19(Suppl 4):iv39–iv40. doi:10.1093/annonc/mdn192
Calin GA, Croce CM (2006) MicroRNA signatures in human cancers. Nat Rev Cancer 6:857–866. doi:10.1038/nrc1997
Calin GA, Dumitru CD et al (2002) Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci USA 99:15524–15529. doi:10.1073/pnas.242606799
Michael MZ, O’Connor SM, van Holst Pellekaan NG, Young GP, James RJ (2003) Reduced accumulation of specific microRNAs in colorectal neoplasia. Mol Cancer Res 1:882–891
Takamizawa J, Konishi H et al (2004) Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res 64:3753–3756. doi:10.1158/0008-5472.CAN-04-0637
Cimmino A, Calin GA et al (2005) miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci USA 102:13944–13949. doi:10.1073/pnas.0506654102
Mott JL, Kobayashi S, Bronk SF, Gores GJ (2007) mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene 26:6133–6140. doi:10.1038/sj.onc.1210436
He X, He L, Hannon GJ (2007) The guardian’s little helper: microRNAs in the p53 tumor suppressor network. Cancer Res 67:11099–11101. doi:10.1158/0008-5472.CAN-07-2672
Hermeking H (2007) p53 enters the microRNA world. Cancer Cell 12:414–418. doi:10.1016/j.ccr.2007.10.028
Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T (2007) Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet 39:673–677. doi:10.1038/ng2003
Tarasov V, Jung P et al (2007) Differential regulation of microRNAs by p53 revealed by massively parallel sequencing: miR-34a is a p53 target that induces apoptosis and G1-arrest. Cell Cycle 6:1586–1593
Raver-Shapira N, Marciano E et al (2007) Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol Cell 26:731–743. doi:10.1016/j.molcel.2007.05.017
Corney DC, Flesken-Nikitin A, Godwin AK, Wang W, Nikitin AY (2007) MicroRNA-34b and MicroRNA-34c are targets of p53 and cooperate in control of cell proliferation and adhesion-independent growth. Cancer Res 67:8433–8438. doi:10.1158/0008-5472.CAN-07-1585
Bommer GT, Gerin I et al (2007) p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr Biol 17:1298–1307. doi:10.1016/j.cub.2007.06.068
Welch C, Chen Y, Stallings RL (2007) MicroRNA-34a functions as a potential tumor suppressor by inducing apoptosis in neuroblastoma cells. Oncogene 26:5017–5022. doi:10.1038/sj.onc.1210293
Brugarolas J, Chandrasekaran C et al (1995) Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature 377:552–557. doi:10.1038/377552a0
Yamakuchi M, Ferlito M, Lowenstein CJ (2008) miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci USA 105:13421–13426. doi:10.1073/pnas.0801613105
Ji Q, Hao X et al (2008) Restoration of tumor suppressor miR-34 inhibits human p53-mutant gastric cancer tumorspheres. BMC Cancer 8:266. doi:10.1186/1471-2407-8-266
Eis PS, Tam W et al (2005) Accumulation of miR-155 and BIC RNA in human B cell lymphomas. Proc Natl Acad Sci USA 102:3627–3632. doi:10.1073/pnas.0500613102
Shi XB, Xue L et al (2007) An androgen-regulated miRNA suppresses Bak1 expression and induces androgen-independent growth of prostate cancer cells. Proc Natl Acad Sci USA 104:19983–19988. doi:10.1073/pnas.0706641104
Weissman IL (2000) Stem cells: units of development, units of regeneration, and units in evolution. Cell 100:157–168. doi:10.1016/S0092-8674(00)81692-X
Gazit R, Weissman IL, Rossi DJ (2008) Hematopoietic stem cells and the aging hematopoietic system. Semin Hematol 45:218–224. doi:10.1053/j.seminhematol.2008.07.010
Rossi DJ, Jamieson CH, Weissman IL (2008) Stems cells and the pathways to aging and cancer. Cell 132:681–696. doi:10.1016/j.cell.2008.01.036
Soltysova A, Altanerova V, Altaner C (2005) Cancer stem cells. Neoplasma 52:435–440
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF (2003) Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 100:3983–3988. doi:10.1073/pnas.0530291100
Singh SK, Hawkins C et al (2004) Identification of human brain tumour initiating cells. Nature 432:396–401. doi:10.1038/nature03128
Calabrese C, Poppleton H et al (2007) A perivascular niche for brain tumor stem cells. Cancer Cell 11:69–82. doi:10.1016/j.ccr.2006.11.020
Ogilvy S, Metcalf D et al (1999) Constitutive Bcl-2 expression throughout the hematopoietic compartment affects multiple lineages and enhances progenitor cell survival. Proc Natl Acad Sci USA 96:14943–14948. doi:10.1073/pnas.96.26.14943
Domen J, Gandy KL, Weissman IL (1998) Systemic overexpression of BCL-2 in the hematopoietic system protects transgenic mice from the consequences of lethal irradiation. Blood 91:2272–2282
Mekori YA, Gilfillan AM, Akin C, Hartmann K, Metcalfe DD (2001) Human mast cell apoptosis is regulated through Bcl-2 and Bcl-XL. J Clin Immunol 21:171–174. doi:10.1023/A:1011083031272
Nusslein-Volhard C, Wieschaus E (1980) Mutations affecting segment number and polarity in Drosophila. Nature 287:795–801. doi:10.1038/287795a0
Ruiz i Altaba A, Sanchez P, Dahmane N (2002) Gli and hedgehog in cancer: tumours, embryos and stem cells. Nat Rev Cancer 2:361–372. doi:10.1038/nrc796
Beachy PA, Karhadkar SS, Berman DM (2004) Tissue repair and stem cell renewal in carcinogenesis. Nature 432:324–331. doi:10.1038/nature03100
Rodriguez-Villanueva J, Colome MI, Brisbay S, McDonnell TJ (1995) The expression and localization of bcl-2 protein in normal skin and in non-melanoma skin cancers. Pathol Res Pract 191:391–398
Ghali L, Wong ST, Green J, Tidman N, Quinn AG (1999) Gli1 protein is expressed in basal cell carcinomas, outer root sheath keratinocytes and a subpopulation of mesenchymal cells in normal human skin. J Invest Dermatol 113:595–599. doi:10.1046/j.1523-1747.1999.00729.x
Bigelow RL, Chari NS et al (2004) Transcriptional regulation of bcl-2 mediated by the sonic hedgehog signaling pathway through gli-1. J Biol Chem 279:1197–1205. doi:10.1074/jbc.M310589200
Qin H, Yu T et al (2007) Regulation of apoptosis and differentiation by p53 in human embryonic stem cells. J Biol Chem 282:5842–5852. doi:10.1074/jbc.M610464200
Meletis K, Wirta V et al (2006) p53 suppresses the self-renewal of adult neural stem cells. Development 133:363–369. doi:10.1242/dev.02208
Aladjem MI, Spike BT et al (1998) ES cells do not activate p53-dependent stress responses and undergo p53-independent apoptosis in response to DNA damage. Curr Biol 8:145–155. doi:10.1016/S0960-9822(98)70061-2
Lin T, Chao C et al (2005) p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat Cell Biol 7:165–171. doi:10.1038/ncb1211
Maimets T, Neganova I, Armstrong L, Lako M (2008) Activation of p53 by nutlin leads to rapid differentiation of human embryonic stem cells. Oncogene 27:5277–5287. doi:10.1038/onc.2008.166
Valentin-Vega YA, Okano H, Lozano G (2008) The intestinal epithelium compensates for p53-mediated cell death and guarantees organismal survival. Cell Death Differ 15:1772–1781. doi:10.1038/cdd.2008.109
Spurgers KB, Chari NS, Bohnenstiehl NL, McDonnell TJ (2006) Molecular mediators of cell death in multistep carcinogenesis: a path to targeted therapy. Cell Death Differ 13:1360–1370. doi:10.1038/sj.cdd.4401986
Shangary S, Wang S (2008) Targeting the MDM2–p53 interaction for cancer therapy. Clin Cancer Res 14:5318–5324. doi:10.1158/1078-0432.CCR-07-5136
Zeitlin BD, Zeitlin IJ, Nor JE (2008) Expanding circle of inhibition: small-molecule inhibitors of Bcl-2 as anticancer cell and antiangiogenic agents. J Clin Oncol 26:4180–4188. doi:10.1200/JCO.2007.15.7693
Chari NS, McDonnell TJ (2007) The sonic hedgehog signaling network in development and neoplasia. Adv Anat Pathol 14:344–352. doi:10.1097/PAP.0b013e3180ca8a1d
Karhadkar SS, Bova GS et al (2004) Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature 431:707–712. doi:10.1038/nature02962
Chen JK, Taipale J, Cooper MK, Beachy PA (2002) Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev 16:2743–2748. doi:10.1101/gad.1025302
Chen JK, Taipale J, Young KE, Maiti T, Beachy PA (2002) Small molecule modulation of smoothened activity. Proc Natl Acad Sci USA 99:14071–14076. doi:10.1073/pnas.182542899
Romer JT, Kimura H et al (2004) Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/−)p53(−/−) mice. Cancer Cell 6:229–240. doi:10.1016/j.ccr.2004.08.019
Zhang Y, Laterra J, Pomper MG (2009) Hedgehog pathway inhibitor HhAntag691 is a potent inhibitor of ABCG2/BCRP and ABCB1/Pgp. Neoplasia 11:96–101
Fukumoto T, Watanabe-Fukunaga R, Fujisawa K, Nagata S, Fukunaga R (2001) The fused protein kinase regulates Hedgehog-stimulated transcriptional activation in Drosophila Schneider 2 cells. J Biol Chem 276:38441–38448. doi:10.1074/jbc.M105871200
Kise Y, Takenaka K, Tezuka T, Yamamoto T, Miki H (2006) Fused kinase is stabilized by Cdc37/Hsp90 and enhances Gli protein levels. Biochem Biophys Res Commun 351:78–84. doi:10.1016/j.bbrc.2006.10.036
Roth JA (1998) Restoration of tumour suppressor gene expression for cancer. Forum (Genova) 8:368–376
Kawabe S, Munshi A et al (2001) Adenovirus-mediated wild-type p53 gene expression radiosensitizes non-small cell lung cancer cells but not normal lung fibroblasts. Int J Radiat Biol 77:185–194. doi:10.1080/09553000010008540
Chada S, Menander KB, Bocangel D, Roth JA, Ramesh R (2008) Cancer targeting using tumor suppressor genes. Front Biosci 13:1959–1967. doi:10.2741/2815
Gabrilovich DI (2006) INGN 201 (Advexin): adenoviral p53 gene therapy for cancer. Expert Opin Biol Ther 6:823–832. doi:10.1517/14712598.6.8.823
Eastham JA, Grafton W, Martin CM, Williams BJ (2000) Suppression of primary tumor growth and the progression to metastasis with p53 adenovirus in human prostate cancer. J Urol 164:814–819. doi:10.1016/S0022-5347(05)67320-X
Inoue A, Narumi K et al (2000) Administration of wild-type p53 adenoviral vector synergistically enhances the cytotoxicity of anti-cancer drugs in human lung cancer cells irrespective of the status of p53 gene. Cancer Lett 157:105–112. doi:10.1016/S0304-3835(00)00480-8
Bischoff JR, Kirn DH et al (1996) An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science 274:373–376. doi:10.1126/science.274.5286.373
Bouchet BP, de Fromentel CC, Puisieux A, Galmarini CM (2006) p53 as a target for anti-cancer drug development. Crit Rev Oncol Hematol 58:190–207. doi:10.1016/j.critrevonc.2005.10.005
Marine JC, Francoz S et al (2006) Keeping p53 in check: essential and synergistic functions of Mdm2 and Mdm4. Cell Death Differ 13:927–934. doi:10.1038/sj.cdd.4401912
Vassilev LT, Vu BT et al (2004) In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303:844–848. doi:10.1126/science.1092472
Issaeva N, Bozko P et al (2004) Small molecule RITA binds to p53, blocks p53-HDM-2 interaction and activates p53 function in tumors. Nat Med 10:1321–1328. doi:10.1038/nm1146
Hu CQ, Hu YZ (2008) Small molecule inhibitors of the p53-MDM2. Curr Med Chem 15:1720–1730. doi:10.2174/092986708784872375
Murray JK, Gellman SH (2007) Targeting protein–protein interactions: lessons from p53/MDM2. Biopolymers 88:657–686. doi:10.1002/bip.20741
Shangary, S. and S. Wang (2008) Small-molecule inhibitors of the MDM2-p53 protein–protein interaction to reactivate p53 function: a novel approach for cancer therapy. Annu Rev Pharmacol Toxicol [Epub ahead of print]
Zhivotovsky B, Orrenius S (2006) Carcinogenesis and apoptosis: paradigms and paradoxes. Carcinogenesis 27:1939–1945. doi:10.1093/carcin/bgl035
Delehedde M, Cho SH et al (1999) Altered expression of bcl-2 family member proteins in nonmelanoma skin cancer. Cancer 85:1514–1522
Rassidakis GZ, Lai R et al (2002) Overexpression of Mcl-1 in anaplastic large cell lymphoma cell lines and tumors. Am J Pathol 160:2309–2310
Williams J, Lucas PC et al (2005) Expression of Bcl-xL in ovarian carcinoma is associated with chemoresistance and recurrent disease. Gynecol Oncol 96:287–295. doi:10.1016/j.ygyno.2004.10.026
Minn AJ, Rudin CM, Boise LH, Thompson CB (1995) Expression of bcl-xL can confer a multidrug resistance phenotype. Blood 86:1903–1910
Miyake H, Tolcher A, Gleave ME (1999) Antisense Bcl-2 oligodeoxynucleotides inhibit progression to androgen-independence after castration in the Shionogi tumor model. Cancer Res 59:4030–4034
Piro LD (2004) Apoptosis, Bcl-2 antisense, and cancer therapy. Oncology (Williston Park) 18:5–10
Liu X, Dai S, Zhu Y, Marrack P, Kappler JW (2003) The structure of a Bcl-xL/Bim fragment complex: implications for Bim function. Immunity 19:341–352. doi:10.1016/S1074-7613(03)00234-6
Day CL, Chen L et al (2005) Solution structure of prosurvival Mcl-1 and characterization of its binding by proapoptotic BH3-only ligands. J Biol Chem 280:4738–4744. doi:10.1074/jbc.M411434200
Sattler M, Liang H et al (1997) Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science 275:983–986. doi:10.1126/science.275.5302.983
Oltersdorf T, Elmore SW et al (2005) An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435:677–681. doi:10.1038/nature03579
Fesik SW (2005) Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer 5:876–885. doi:10.1038/nrc1736
Letai AG (2008) Diagnosing and exploiting cancer’s addiction to blocks in apoptosis. Nat Rev Cancer 8:121–132. doi:10.1038/nrc2297
Acknowledgments
This work was supported by The Mehta Family Foundation and NCI UO1 CA105345. NP is supported by the Department of Defense PC074107 and a Sowell-Huggins Predoctoral Fellowship. TJM is the recipient of the Sowell-Huggins Professorship in Cancer Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chari, N.S., Pinaire, N.L., Thorpe, L. et al. The p53 tumor suppressor network in cancer and the therapeutic modulation of cell death. Apoptosis 14, 336–347 (2009). https://doi.org/10.1007/s10495-009-0327-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-009-0327-9