Abstract
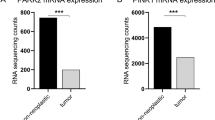
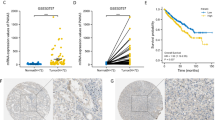
Activation of the initiator-caspase, caspase-8 is under tight control of multiple antiapoptotic regulators including ARC, cFlipS, cFlipL and PED/PEA-15. Since there is little data regarding the expression of caspase-8 and its antiapoptotic regulators in human tumours in vivo, we analysed their expression in renal cell carcinomas (RCCs) to identify which of these genes might be crucial for the well known impaired apoptosis and—as a result—resistance towards chemotherapy and ionizing radiation of RCCs. Caspase-8, cFlipS, cFlipL and PED/PEA-15 mRNA expression was significantly increased only in early stages of RCCs compared to non-neoplastic renal tissue. In contrast, ARC mRNA expression was significantly increased in RCCs of all stages without differences between the tumour stages and grades. Importantly, the relative mRNA expression ratio between ARC and caspase-8 was significantly increased during carcinogenesis and tumour progression. In contrast, the relative mRNA expression ratio between cFlipS, cFlipL or PED/PEA-15 and caspase-8 remained constant during all tumour stages. In conclusion, our analysis revealed that ARC is the only caspase-8 inhibiting regulator being constantly overexpressed in RCCs. Furthermore, the balance between antiapoptotic ARC and proapoptotic caspase-8 is the only one to be disturbed during carcinogenesis and tumour progression of RCCs. This inhibition of Caspase-8 might therefore be one example for the multiple antiapoptotic functions of ARC in RCCs possibly contributing to the marked resistance of RCCs towards radio- and chemotherapy and reflects a shift of gene expression towards a more antiapoptotic context in RCCs.








Similar content being viewed by others
References
Gerharz CD, Ramp U, Dejosez M et al (1999) Resistance to CD95 (APO-1/Fas)-mediated apoptosis in human renal cell carcinomas: an important factor for evasion from negative growth control. Lab Invest 79:1521–1534
Ramp U, Dejosez M, Mahotka C et al (2000) Deficient activation of CD95 (APO-1/Fas)-mediated apoptosis: a potential factor of multidrug resistance in human renal cell carcinoma. Br J Cancer 82:1851–1859. doi:10.1054/bjoc.2000.1155
Wang S, El-Deiry WS (2003) TRAIL and apoptosis induction by TNF-family death receptors. Oncogene 22:8628–8633. doi:10.1038/sj.onc.1207232
Wu GS, Burns TF, McDonald ER 3rd et al (1997) KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nat Genet 17:141–143. doi:10.1038/ng1097–141
Owen-Schaub LB, Zhang W, Cusack JC et al (1995) Wild-type human p53 and a temperature-sensitive mutant induce Fas/APO-1 expression. Mol Cell Biol 15:3032–3040
Vakifahmetoglu H, Olsson M, Orrenius S, Zhivotovsky B (2006) Functional connection between p53 and caspase-2 is essential for apoptosis induced by DNA damage. Oncogene 25:5683–5692. doi:10.1038/sj.onc.1209569
Lin CF, Chen CL, Chang WT et al (2004) Sequential caspase-2 and caspase-8 activation upstream of mitochondria during ceramide and etoposide-induced apoptosis. J Biol Chem 279:40755–40761. doi:10.1074/jbc.M404726200
Koseki T, Inohara N, Chen S, Nunez G (1998) ARC, an inhibitor of apoptosis expressed in skeletal muscle and heart that interacts selectively with caspases. Proc Natl Acad Sci USA 95:5156–5160. doi:10.1073/pnas.95.9.5156
Ekhterae D, Lin Z, Lundberg MS, Crow MT, Brosius FC 3rd, Nunez G (1999) ARC inhibits cytochrome c release from mitochondria and protects against hypoxia-induced apoptosis in heart-derived H9c2 cells. Circ Res 85:e70–e77
Abmayr S, Crawford RW, Chamberlain JS (2004) Characterization of ARC, apoptosis repressor interacting with CARD, in normal and dystrophin-deficient skeletal muscle. Hum Mol Genet 13:213–221. doi:10.1093/hmg/ddh018
Wang M, Qanungo S, Crow MT, Watanabe M, Nieminen AL (2005) Apoptosis repressor with caspase recruitment domain (ARC) is expressed in cancer cells and localizes to nuclei. FEBS Lett 579:2411–2415. doi:10.1016/j.febslet.2005.03.040
Neuss M, Monticone R, Lundberg MS, Chesley AT, Fleck E, Crow MT (2001) The apoptotic regulatory protein ARC (apoptosis repressor with caspase recruitment domain) prevents oxidant stress-mediated cell death by preserving mitochondrial function. J Biol Chem 276:33915–33922. doi:10.1074/jbc.M104080200
Gustafsson AB, Tsai JG, Logue SE, Crow MT, Gottlieb RA (2004) Apoptosis repressor with caspase recruitment domain protects against cell death by interfering with Bax activation. J Biol Chem 279:21233–21238. doi:10.1074/jbc.M400695200
Nam YJ, Mani K, Ashton AW et al (2004) Inhibition of both the extrinsic and intrinsic death pathways through nonhomotypic death-fold interactions. Mol Cell 15:901–912. doi:10.1016/j.molcel.2004.08.020
Foo RS, Nam YJ, Ostreicher MJ et al (2007) Regulation of p53 tetramerization and nuclear export by ARC. Proc Natl Acad Sci USA 104:20826–20831. doi:10.1073/pnas.0710017104
Mercier I, Vuolo M, Madan R et al (2005) ARC, an apoptosis suppressor limited to terminally differentiated cells, is induced in human breast cancer and confers chemo- and radiation-resistance. Cell Death Differ 12:682–686. doi:10.1038/sj.cdd.4401631
Zhang YQ, Herman B Expression, modification of ARC (apoptosis repressor with a CARD domain) is distinctly regulated by oxidative stress in cancer cells. J Cell Biochem 2008 (Epub ahead of print)
Renault F, Formstecher E, Callebaut I, Junier MP, Chneiweiss H (2003) The multifunctional protein PEA-15 is involved in the control of apoptosis and cell cycle in astrocytes. Biochem Pharmacol 66:1581–1588. doi:10.1016/S0006-2952(03)00514-8
Ricci-Vitiani L, Pedini F, Mollinari C et al (2004) Absence of caspase 8 and high expression of PED protect primitive neural cells from cell death. J Exp Med 200:1257–1266. doi:10.1084/jem.20040921
Condorelli G, Vigliotta G, Iavarone C et al (1998) PED/PEA-15 gene controls glucose transport and is overexpressed in type 2 diabetes mellitus. EMBO J 17:3858–3866. doi:10.1093/emboj/17.14.3858
Condorelli G, Vigliotta G, Cafieri A et al (1999) PED/PEA-15: an anti-apoptotic molecule that regulates FAS/TNFR1-induced apoptosis. Oncogene 18:4409–4415. doi:10.1038/sj.onc.1202831
Trencia A, Fiory F, Maitan MA et al (2004) Omi/HtrA2 promotes cell death by binding and degrading the anti-apoptotic protein ped/pea-15. J Biol Chem 279:46566–46572. doi:10.1074/jbc.M406317200
Condorelli G, Trencia A, Vigliotta G et al (2002) Multiple members of the mitogen-activated protein kinase family are necessary for PED/PEA-15 anti-apoptotic function. J Biol Chem 277:11013–11018. doi:10.1074/jbc.M110934200
Hao C, Beguinot F, Condorelli G et al (2001) Induction and intracellular regulation of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) mediated apoptosis in human malignant glioma cells. Cancer Res 61:1162–1170
Xiao C, Yang BF, Asadi N, Beguinot F, Hao C (2002) Tumor necrosis factor-related apoptosis-inducing ligand-induced death-inducing signaling complex and its modulation by c-FLIP and PED/PEA-15 in glioma cells. J Biol Chem 277:25020–25025. doi:10.1074/jbc.M202946200
Formisano P, Perruolo G, Libertini S et al (2005) Raised expression of the antiapoptotic protein ped/pea-15 increases susceptibility to chemically induced skin tumor development. Oncogene 24:7012–7021. doi:10.1038/sj.onc.1208871
Glading A, Koziol JA, Krueger J, Ginsberg MH (2007) PEA-15 inhibits tumor cell invasion by binding to extracellular signal-regulated kinase 1/2. Cancer Res 67:1536–1544. doi:10.1158/0008-5472.CAN-06-1378
Scaffidi C, Schmitz I, Krammer PH, Peter ME (1999) The role of c-FLIP in modulation of CD95-induced apoptosis. J Biol Chem 274:1541–1548. doi:10.1074/jbc.274.3.1541
Clarke P, Tyler KL (2007) Down-regulation of cFLIP following reovirus infection sensitizes human ovarian cancer cells to TRAIL-induced apoptosis. Apoptosis 12:211–223. doi:10.1007/s10495-006-0528-4
White SJ, Lu P, Keller GM, Voelkel-Johnson C (2006) Targeting the short form of cFLIP by RNA interference is sufficient to enhance TRAIL sensitivity in PC3 prostate carcinoma cells. Cancer Biol Ther 5:1618–1623
Lee TJ, Lee JT, Park JW, Kwon TK (2006) Acquired TRAIL resistance in human breast cancer cells are caused by the sustained cFLIP(L) and XIAP protein levels and ERK activation. Biochem Biophys Res Commun 351:1024–1030. doi:10.1016/j.bbrc.2006.10.163
El-Zawahry A, Lu P, White SJ, Voelkel-Johnson C (2006) In vitro efficacy of AdTRAIL gene therapy of bladder cancer is enhanced by trichostatin A-mediated restoration of CAR expression and downregulation of cFLIP and Bcl-XL. Cancer Gene Ther 13:281–289. doi:10.1038/sj.cgt.7700905
Brooks AD, Sayers TJ (2005) Reduction of the antiapoptotic protein cFLIP enhances the susceptibility of human renal cancer cells to TRAIL apoptosis. Cancer Immunol Immunother 54:499–505. doi:10.1007/s00262-004-0595-8
Korkolopoulou P, Goudopoulou A, Voutsinas G et al (2004) c-FLIP expression in bladder urothelial carcinomas: its role in resistance to Fas-mediated apoptosis and clinicopathologic correlations. Urology 63:1198–1204. doi:10.1016/j.urology.2004.01.007
Dolcet X, Llobet D, Pallares J, Rue M, Comella JX, Matias-Guiu X (2005) FLIP is frequently expressed in endometrial carcinoma and has a role in resistance to TRAIL-induced apoptosis. Lab Invest 85:885–894. doi:10.1038/labinvest.3700286
Chen HX, Liu YJ, Zhou XD, Luo RY (2005) Expression of cellular FLICE/caspase-8 inhibitory protein is associated with malignant potential in endometrial carcinoma. Int J Gynecol Cancer 15:663–670. doi:10.1111/j.1525-1438.2005.00122.x
Thomas RK, Kallenborn A, Wickenhauser C et al (2002) Constitutive expression of c-FLIP in Hodgkin and Reed-Sternberg cells. Am J Pathol 160:1521–1528
Uherova P, Olson S, Thompson MA, Juskevicius R, Hamilton KS (2004) Expression of c-FLIP in classic and nodular lymphocyte-predominant Hodgkin lymphoma. Appl Immunohistochem Mol Morphol 12:105–110. doi:10.1097/00129039-200406000-00002
Ryu BK, Lee MG, Chi SG, Kim YW, Park JH (2001) Increased expression of cFLIP(L) in colonic adenocarcinoma. J Pathol 194:15–19. doi:10.1002/path.835
Lee SH, Kim HS, Kim SY et al (2003) Increased expression of FLIP, an inhibitor of Fas-mediated apoptosis, in stomach cancer. APMIS 111:309–314. doi:10.1034/j.1600-0463.2003.1110203.x
Zhou XD, Yu JP, Liu J, Luo HS, Chen HX, Yu HG (2004) Overexpression of cellular FLICE-inhibitory protein (FLIP) in gastric adenocarcinoma. Clin Sci (Lond) 106:397–405. doi:10.1042/CS20030238
Bullani RR, Huard B, Viard-Leveugle I, Byers HR, Irmler M, Saurat JH et al (2001) Selective expression of FLIP in malignant melanocytic skin lesions. J Invest Dermatol 117:360–364. doi:10.1046/j.0022-202x.2001.01418.x
Valnet-Rabier MB, Challier B, Thiebault S et al (2005) c-Flip protein expression in Burkitt’s lymphomas is associated with a poor clinical outcome. Br J Haematol 128:767–773. doi:10.1111/j.1365-2141.2005.05378.x
Valente G, Manfroi F, Peracchio C et al (2006) cFLIP expression correlates with tumour progression and patient outcome in non-Hodgkin lymphomas of low grade of malignancy. Br J Haematol 132:560–570. doi:10.1111/j.1365-2141.2005.05898.x
Eckelman BP, Salvesen GS, Scott FL (2006) Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep 7:988–994. doi:10.1038/sj.embor.7400795
Ramp U, Krieg T, Caliskan E et al (2004) XIAP expression is an independent prognostic marker in clear-cell renal carcinomas. Hum Pathol 35:1022–1028. doi:10.1016/j.humpath.2004.03.011
Shibata T, Mahotka C, Wethkamp N, Heikaus S, Gabbert HE, Ramp U (2007) Disturbed expression of the apoptosis regulators XIAP, XAF1, and Smac/DIABLO in gastric adenocarcinomas. Diagn Mol Pathol 16:1–8. doi:10.1097/01.pdm.0000213471.92925.51
Boatright KM, Deis C, Denault JB, Sutherlin DP, Salvesen GS (2004) Activation of caspases-8 and -10 by FLIP(L). Biochem J 382:651–657. doi:10.1042/BJ20040809
Micheau O, Thome M, Schneider P et al (2002) The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. J Biol Chem 277:45162–45171. doi:10.1074/jbc.M206882200
Chang DW, Xing Z, Pan Y et al (2002) c-FLIP(L) is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. EMBO J 21:3704–3714. doi:10.1093/emboj/cdf356
Higuchi H, Yoon JH, Grambihler A, Werneburg N, Bronk SF, Gores GJ (2003) Bile acids stimulate cFLIP phosphorylation enhancing TRAIL-mediated apoptosis. J Biol Chem 278:454–461. doi:10.1074/jbc.M209387200
Acknowledgement
This work was supported by the “Stiftung fuer Altersforschung” of the Heinrich-Heine University, Duesseldorf.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Heikaus, S., Kempf, T., Mahotka, C. et al. Caspase-8 and its inhibitors in RCCs in vivo: the prominent role of ARC. Apoptosis 13, 938–949 (2008). https://doi.org/10.1007/s10495-008-0225-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-008-0225-6