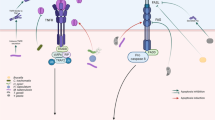
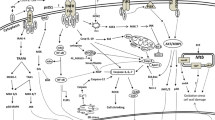
Abstract
Apoptosis, or programmed cell death, is normally responsible for the orderly elimination of aged or damaged cells, and is a necessary part of the homeostasis and development of multicellular organisms. Some pathogenic bacteria can disrupt this process by triggering excess apoptosis or by preventing it when appropriate. Either event can lead to disease. There has been extensive research into the modulation of host cell death by microorganisms, and several reviews have been published on the phenomenon. Rather than covering the entire field, this review focuses on the dysregulation of host cell apoptosis by members of the order Actinomycetales, containing the genera Corynebacterium, Mycobacterium, Rhodococcus, and Nocardia.
Similar content being viewed by others
References
DeLeo FR (2004) Modulation of phagocyte apoptosis by bacterial pathogens. Apoptosis 9:399–413
Gao L, Abu Kwaik Y (2000) Hijacking of apoptotic pathways by bacterial pathogens. Microbes Infect 2:1705–1719
Moss JE, Aliprantis AO, Zychlinsky A (1999) The regulation of apoptosis by microbial pathogens. Int Rev Cytol 187:203–259
Weinrauch Y, Zychlinsky A (1999) The induction of apoptosis by bacterial pathogens. Annu Rev Microbiol 53:155–187
Raffray M, Cohen GM (1997) Apoptosis and necrosis in toxicology: a continuum or distinct modes of cell death? Pharmacol Ther 75:153–177
Edinger AL, Thompson CB (2004) Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol 16:663–669
Rossi D, Gaidano G (2003) Messengers of cell death: apoptotic signaling in health and disease. Haematologica 88:212–218
Cande C, Cecconi F, Dessen P, Kroemer G (2002) Apoptosis-inducing factor (AIF): key to the conserved caspase-independent pathways of cell death? J Cell Sci 115:4727–4734
Schwartz DA, Wharton M. Diphtheria. (1998) In: Horsburgh CR, Nelson AM, (eds) Pathology of emerging infections 2. ASM Press, Washington D.C., pp 145–166
Chen Y, Zychlinsky A (1994) Apoptosis induced by bacterial pathogens. Microb Pathog 17:203–212
Kusano I, Kageyama A, Tamura T, Oda T, Muramatsu T (2001) Enhancement of diphtheria toxin-induced apoptosis in Vero cells by combination treatment with brefeldin A and okadaic acid. Cell Struct Funct 26:279–288
Lesieur C, Vecsey-Semjen B, Abrami L, Fivaz M, Gisou van der Goot F (1997) Membrane insertion: the strategies of toxins (review). Mol Membr Biol 14:45–64
Komatsu N, Oda T, Muramatsu T (1998) Involvement of both caspase-like proteases and serine proteases in apoptotic cell death induced by ricin, modeccin, diphtheria toxin, and pseudomonas toxin. J Biochem (Tokyo) 124:1038–1044
Brinkmann U, Mansfield E, Pastan I (1997) Effects of BCL-2 overexpression on the sensitivity of MCF-7 breast cancer cells to ricin, diphtheria and Pseudomonas toxin and immunotoxins. Apoptosis 2:192–198
Thorburn J, Frankel AE, Thorburn A (2003) Apoptosis by leukemia cell-targeted diphtheria toxin occurs via receptor-independent activation of Fas-associated death domain protein. Clin Cancer Res 9:861–865
Kochi SK, Collier RJ (1993) DNA fragmentation and cytolysis in U937 cells treated with diphtheria toxin or other inhibitors of protein synthesis. Exp Cell Res 208:296–302
Chang MP, Bramhall J, Graves S, Bonavida B, Wisnieski BJ (1989) Internucleosomal DNA cleavage precedes diphtheria toxin-induced cytolysis. Evidence that cell lysis is not a simple consequence of translation inhibition. J Biol Chem 264:15261–15267
Morimoto H, Bonavida B (1992) Diphtheria toxin- and Pseudomonas A toxin-mediated apoptosis. ADP ribosylation of elongation factor-2 is required for DNA fragmentation and cell lysis and synergy with tumor necrosis factor-alpha. J Immunol 149:2089–2094
Sandvig K, van Deurs B (1992) Toxin-induced cell lysis: protection by 3-methyladenine and cycloheximide. Exp Cell Res 200:253–262
Brinkmann U, Brinkmann E, Gallo M, Pastan I (1995) Cloning and characterization of a cellular apoptosis susceptibility gene, the human homologue to the yeast chromosome segregation gene CSE1. Proc Natl Acad Sci USA 92:10427–10431
Brinkmann U, Brinkmann E, Gallo M, Scherf U, Pastan I (1996) Role of CAS, a human homologue to the yeast chromosome segregation gene CSE1, in toxin and tumor necrosis factor mediated apoptosis. Biochemistry 35:6891–6899
Kageyama A, Kusano I, Tamura T, Oda T, Muramatsu T (2002) Comparison of the apoptosis-inducing abilities of various protein synthesis inhibitors in U937 cells. Biosci Biotechnol Biochem 66:835–839
Chang MP, Baldwin RL, Bruce C, Wisnieski BJ (1989) Second cytotoxic pathway of diphtheria toxin suggested by nuclease activity. Science 246:1165–1168
Brinkmann U, Brinkmann E, Pastan I (1995) Expression cloning of cDNAs that render cancer cells resistant to Pseudomonas and diphtheria toxin and immunotoxins. Mol Med 1:206–216
Oddo M, Renno T, Attinger A et al. (1998) Fas ligand-induced apoptosis of infected human macrophages reduces the viability of intracellular Mycobacterium tuberculosis. J Immunol 160:5448–5454
Duan L, Gan H, Golan DE, Remold HG (2002) Critical role of mitochondrial damage in determining outcome of macrophage infection with Mycobacterium tuberculosis. J Immunol 169:5181–5187
Keane J, Shurtleff B, Kornfeld H (2002) TNF-dependent BALB/c murine macrophage apoptosis following Mycobacterium tuberculosis infection inhibits bacillary growth in an IFN-gamma independent manner. Tuberculosis (Edinb) 82:55–61
Lopez M, Sly LM, Luu Y et al. (2003) The 19-kDa Mycobacterium tuberculosis protein induces macrophage apoptosis through Toll-like receptor-2. J Immunol 170:2409–2416
Sly LM, Hingley-Wilson SM, Reiner NE, McMaster WR (2003) Survival of Mycobacterium tuberculosis in host macrophages involves resistance to apoptosis dependent upon induction of antiapoptotic Bcl-2 family member Mcl-1. J Immunol 170:430–437
Keane J, Balcewicz-Sablinska MK, Remold HG et al. (1997) Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis. Infect Immun 65:298–304
Balcewicz-Sablinska MK, Keane J, Kornfeld H, Remold HG (1998) Pathogenic Mycobacterium tuberculosis evades apoptosis of host macrophages by release of TNF-R2, resulting in inactivation of TNF-alpha. J Immunol 161:2636–2641
Keane J, Remold HG, Kornfeld H (2000) Virulent Mycobacterium tuberculosis strains evade apoptosis of infected alveolar macrophages. J Immunol 164:2016–2020
Danelishvili L, McGarvey J, Li YJ, Bermudez LE (2003) Mycobacterium tuberculosis infection causes different levels of apoptosis and necrosis in human macrophages and alveolar epithelial cells. Cell Microbiol 5:649–660
Riendeau CJ, Kornfeld H (2003) THP-1 cell apoptosis in response to mycobacterial infection. Infect Immun 71:254–259
Spira A, Carroll JD, Liu G et al. (2003) Apoptosis genes in human alveolar macrophages infected with virulent or attenuated Mycobacterium tuberculosis: a pivotal role for tumor necrosis factor. Am J Respir Cell Mol Biol 29:545–551
Rojas M, Barrera LF, Puzo G, Garcia LF (1997) Differential induction of apoptosis by virulent Mycobacterium tuberculosis in resistant and susceptible murine macrophages: role of nitric oxide and mycobacterial products. J Immunol 159:1352–1361
Abarca-Rojano E, Rosas-Medina P, Zamudio-Cortez P, Mondragon-Flores R, Sanchez-Garcia FJ (2003) Mycobacterium tuberculosis virulence correlates with mitochondrial cytochrome c release in infected macrophages. Scand J Immunol 58:419–427
Perskvist N, Long M, Stendahl O, Zheng L (2002) Mycobacterium tuberculosis promotes apoptosis in human neutrophils by activating caspase-3 and altering expression of Bax/Bcl-xL via an oxygen-dependent pathway. J Immunol 168:6358–6365
Watson VE, Hill LL, Owen-Schaub LB et al. (2000) Apoptosis in Mycobacterium tuberculosis infection in mice exhibiting varied immunopathology. J Pathol 190:211–220
Placido R, Mancino G, Amendola A et al. (1997) Apoptosis of human monocytes/macrophages in Mycobacterium tuberculosis infection. J Pathol 181:31–38
Ragno S, Estrada-Garcia I, Butler R, Colston MJ (1998) Regulation of macrophage gene expression by Mycobacterium tuberculosis: down-regulation of mitochondrial cytochrome c oxidase. Infect Immun 66:3952–3958
Nuzzo I, Galdiero M, Bentivoglio C, Galdiero R, Romano Carratelli C (2002) Apoptosis modulation by mycolic acid, tuberculostearic acid and trehalose 6,6’-dimycolate. J Infect 44:229–235
Klingler K, Tchou-Wong KM, Brandli O et al. (1997) Effects of mycobacteria on regulation of apoptosis in mononuclear phagocytes. Infect Immun 65:5272–5278
Das SD, Subramanian D, Prabha C (2004) Cell proliferation and apoptosis: dual-signal hypothesis tested in tuberculous pleuritis using mycobacterial antigens. FEMS Immunol Med Microbiol 41:85–92
Rojas M, Olivier M, Garcia LF (2002) Activation of JAK2/STAT1-alpha-dependent signaling events during Mycobacterium tuberculosis-induced macrophage apoptosis. Cell Immunol 217:58–66
Aleman M, Schierloh P, de la Barrera SS et al. (2004) Mycobacterium tuberculosis triggers apoptosis in peripheral neutrophils involving toll-like receptor 2 and p38 mitogen protein kinase in tuberculosis patients. Infect Immun 72:5150–5158
Ciaramella A, Cavone A, Santucci MB et al. (2004) Induction of apoptosis and release of interleukin-1 beta by cell wall-associated 19-kDa lipoprotein during the course of mycobacterial infection. J Infect Dis 190:1167–1176
Ozeki Y, Kaneda K, Fujiwara N et al. (1997) in vivo induction of apoptosis in the thymus by administration of mycobacterial cord factor (trehalose 6,6’-dimycolate). Infect Immun 65:1793–1799
Hamasaki N, Isowa K, Kamada K et al. (2000) in vivo administration of mycobacterial cord factor (Trehalose 6, 6’-dimycolate) can induce lung and liver granulomas and thymic atrophy in rabbits. Infect Immun 68:3704–3709
Ciaramella A, Martino A, Cicconi R, Colizzi V, Fraziano M (2000) Mycobacterial 19-kDa lipoprotein mediates Mycobacterium tuberculosis-induced apoptosis in monocytes/macrophages at early stages of infection. Cell Death Differ 7:1270–1272
Vivekanandhan A, Das S (2001) in vivo study on dual-signal hypothesis of apoptosis using mycobacterial antigen. Curr Sci 81:301–304
Rojas M, Olivier M, Gros P, Barrera LF, Garcia LF (1999) TNF-alpha and IL-10 modulate the induction of apoptosis by virulent Mycobacterium tuberculosis in murine macrophages. J Immunol 162:6122–6131
Gil DP, Leon LG, Correa LI et al. (2004) Differential induction of apoptosis and necrosis in monocytes from patients with tuberculosis and healthy control subjects. J Infect Dis 189:2120–2128
Dao DN, Kremer L, Guerardel Y et al. (2004) Mycobacterium tuberculosis lipomannan induces apoptosis and interleukin-12 production in macrophages. Infect Immun 72:2067–2074
Rojas M, Garcia LF, Nigou J, Puzo G, Olivier M (2000) Mannosylated lipoarabinomannan antagonizes Mycobacterium tuberculosis-induced macrophage apoptosis by altering Ca+2-dependent cell signaling. J Infect Dis 182:240–251
Maiti D, Bhattacharyya A, Basu J (2001) Lipoarabinomannan from Mycobacterium tuberculosis promotes macrophage survival by phosphorylating Bad through a phosphatidylinositol 3-kinase/Akt pathway. J Biol Chem 276:329–333
Guerardel Y, Maes E, Briken V et al. (2003) Lipomannan and lipoarabinomannan from a clinical isolate of Mycobacterium kansasii: novel structural features and apoptosis-inducing properties. J Biol Chem 278:36637–36651
Ghosh S, Pal S, Das S, Dasgupta SK, Majumdar S (1998) Lipoarabinomannan induced cytotoxic effects in human mononuclear cells. FEMS Immunol Med Microbiol 21:181–188
Cree IA, Nurbhai S, Milne G, Beck JS (1987) Cell death in granulomata: the role of apoptosis. J Clin Pathol 40:1314–1319
Mustafa T, Bjune TG, Jonsson R, Pando RH, Nilsen R (2001) Increased expression of fas ligand in human tuberculosis and leprosy lesions: a potential novel mechanism of immune evasion in mycobacterial infection. Scand J Immunol 54:630–639
Hirsch CS, Toossi Z, Vanham G et al. (1999) Apoptosis and T cell hyporesponsiveness in pulmonary tuberculosis. J Infect Dis 179:945–953
Hirsch CS, Toossi Z, Johnson JL et al. (2001) Augmentation of apoptosis and interferon-gamma production at sites of active Mycobacterium tuberculosis infection in human tuberculosis. J Infect Dis 183:779–788
Mustafa T, Phyu S, Nilsen R, Bjune G, Jonsson R (1999) Increased expression of Fas ligand on Mycobacterium tuberculosis infected macrophages: a potential novel mechanism of immune evasion by Mycobacterium tuberculosis ? Inflammation 23:507–521
Ciaramella A, Cavone A, Santucci MB et al. (2002) Proinflammatory cytokines in the course of Mycobacterium tuberculosis-induced apoptosis in monocytes/macrophages. J Infect Dis 186:1277–1282
Duan L, Gan H, Arm J, Remold HG (2001) Cytosolic phospholipase A2 participates with TNF-alpha in the induction of apoptosis of human macrophages infected with Mycobacterium tuberculosis H37Ra. J Immunol 166:7469–7476
Santucci MB, Ciaramella A, Mattei M, Sumerska T, Fraziano M (2003) Batimastat reduces Mycobacterium tuberculosis-induced apoptosis in macrophages. Int Immunopharmacol 3:1657–1665
Means TK, Jones BW, Schromm AB et al. (2001) Differential effects of a Toll-like receptor antagonist on Mycobacterium tuberculosis-induced macrophage responses. J Immunol 166:4074–4082
Li B, Bassiri H, Rossman MD et al. (1998) Involvement of the Fas/Fas ligand pathway in activation-induced cell death of mycobacteria-reactive human gamma delta T cells: a mechanism for the loss of gamma delta T cells in patients with pulmonary tuberculosis. J Immunol 161:1558–1567
Durrbaum-Landmann I, Gercken J, Flad HD, Ernst M (1996) Effect of in vitro infection of human monocytes with low numbers of Mycobacterium tuberculosis bacteria on monocyte apoptosis. Infect Immun 64:5384–5389
McGarvey JA, Wagner D, Bermudez LE (2004) Differential gene expression in mononuclear phagocytes infected with pathogenic and non-pathogenic mycobacteria. Clin Exp Immunol 136:490–500
Mogga SJ, Mustafa T, Sviland L, Nilsen R (2002) Increased Bcl-2 and reduced Bax expression in infected macrophages in slowly progressive primary murine Mycobacterium tuberculosis infection. Scand J Immunol 56:383–391
Briken V, Porcelli SA, Besra GS, Kremer L (2004) Mycobacterial lipoarabinomannan and related lipoglycans: from biogenesis to modulation of the immune response. Mol Microbiol 53:391–403
Rojas M, Barrera LF, Garcia LF (1998) Induction of apoptosis in murine macrophages by Mycobacterium tuberculosis is reactive oxygen intermediates-independent. Biochem Biophys Res Commun 247:436–442
Toossi Z, Hamilton BD, Phillips MH et al. (1997) Regulation of nuclear factor-kappa B and its inhibitor I kappa B-alpha/MAD-3 in monocytes by Mycobacterium tuberculosis and during human tuberculosis. J Immunol 159:4109–4116
Kornfeld H, Mancino G, Colizzi V (1999) The role of macrophage cell death in tuberculosis. Cell Death Differ 6:71–78
Ahmad A, Khan M, Raykundalia C, Catty D (1999) Study of the mechanisms of killing of Mycobacterium bovis BCG by apoptosis in J774 murine macrophages. J Pak Med Assoc 49:273–278
Kremer L, Estaquier J, Wolowczuk I et al. (2000) Ineffective cellular immune response associated with T-cell apoptosis in susceptible Mycobacterium bovis BCG-infected mice. Infect Immun 68:4264–4273
Gutierrez-Pabello JA, McMurray DN, Adams LG (2002) Upregulation of thymosin beta-10 by Mycobacterium bovis infection of bovine macrophages is associated with apoptosis. Infect Immun 70:2121–2127
van Faassen H, Dudani R, Krishnan L, Sad S (2004) Prolonged antigen presentation, APC-, and CD8+ T cell turnover during mycobacterial infection: comparison with Listeria monocytogenes. J Immunol 172:3491–3500
Tokunaga T, Yamamoto H, Shimada S et al. (1984) Antitumor activity of deoxyribonucleic acid fraction from Mycobacterium bovis BCG. I. Isolation, physicochemical characterization, and antitumor activity. J Natl Cancer Inst 72:955–962
Lee CF, Chang SY, Hsieh DS, Yu DS (2004) Treatment of bladder carcinomas using recombinant BCG DNA vaccines and electroporative gene immunotherapy. Cancer Gene Ther 11:194–207
Hersh D, Weiss J, Zychlinsky A (1998) How bacteria initiate inflammation: aspects of the emerging story. Curr Opin Microbiol 1:43–48
Kremer L, Estaquier J, Brandt E, Ameisen JC, Locht C (1997) Mycobacterium bovis Bacillus Calmette Guerin infection prevents apoptosis of resting human monocytes. Eur J Immunol 27:2450–2456
Hall AK (1995) Thymosin beta-10 accelerates apoptosis. Cell Mol Biol Res 41:167–180
Molloy A, Laochumroonvorapong P, Kaplan G. (1994) Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guerin. J Exp Med 180:1499–1509
Horsburgh CR, Nelson AM (1998) Mycobacterium avium. In: Horsburgh CR, Nelson AM, (eds) Pathology of emerging infections 2. ASM Press, Washington D.C., pp 193–216
Gan H, Newman GW, Remold HG (1995) Plasminogen activator inhibitor type 2 prevents programmed cell death of human macrophages infected with Mycobacterium avium, serovar 4. J Immunol 155:1304–1315
Fratazzi C, Arbeit RD, Carini C, Remold HG (1997) Programmed cell death of Mycobacterium avium serovar 4-infected human macrophages prevents the mycobacteria from spreading and induces mycobacterial growth inhibition by freshly added, uninfected macrophages. J Immunol 158:4320–4327
Balcewicz-Sablinska MK, Gan H, Remold HG(1999) Interleukin 10 produced by macrophages inoculated with Mycobacterium avium attenuates mycobacteria-induced apoptosis by reduction of TNF-alpha activity. J Infect Dis 180:1230–1237
Bermudez LE, Parker A, Petrofsky M (1999) Apoptosis of Mycobacterium avium-infected macrophages is mediated by both tumour necrosis factor (TNF) and Fas, and involves the activation of caspases. Clin Exp Immunol 116:94–99
Mohagheghpour N, van Vollenhoven A, Goodman J, Bermudez LE (2000) Interaction of Mycobacterium avium with human monocyte-derived dendritic cells. Infect Immun 68:5824–5829
Allen S, Sotos J, Sylte MJ, Czuprynski CJ (2001) Use of Hoechst 33342 staining to detect apoptotic changes in bovine mononuclear phagocytes infected with Mycobacterium avium subsp. paratuberculosis. Clin Diagn Lab Immunol 8:460–464
Bhattacharyya A, Pathak S, Basak C et al. (2003) Execution of macrophage apoptosis by Mycobacterium avium through apoptosis signal-regulating kinase 1/p38 mitogen-activated protein kinase signaling and caspase 8 activation. J Biol Chem 278:26517–26525
Weiss DJ, Evanson OA, Deng M, Abrahamsen MS (2004) Sequential patterns of gene expression by bovine monocyte-derived macrophages associated with ingestion of mycobacterial organisms. Microb Pathog 37:215–224
Weiss DJ, Evanson OA, Deng M, Abrahamsen MS (2004) Gene expression and antimicrobial activity of bovine macrophages in response to Mycobacterium avium subsp. paratuberculosis. Vet Pathol 41:326–337
Hayashi T, Catanzaro A, Rao SP (1997) Apoptosis of human monocytes and macrophages by Mycobacterium avium sonicate. Infect Immun 65:5262–5271
Gilbertson B, Zhong J, Cheers C (1999) Anergy, IFN-gamma production, and apoptosis in terminal infection of mice with Mycobacterium avium. J Immunol 163:2073–2080
Zhong J, Gilbertson B, Cheers C (2003) Apoptosis of CD4+ and CD8+ T cells during experimental infection with Mycobacterium avium is controlled by Fas/FasL and Bcl-2-sensitive pathways, respectively. Immunol Cell Biol 81:480–486
Cree IA, Gardiner CA, Beck JS, Mehta J (1986) Studies of cell death (apoptosis) and cell division in leprosy granulomas. Int J Lepr Other Mycobact Dis 54:607–613
Walsh DS, Lane JE, Abalos RM, Myint KS (2004) TUNEL and limited immunophenotypic analyses of apoptosis in paucibacillary and multibacillary leprosy lesions. FEMS Immunol Med Microbiol 41:265–269
Hernandez MO, Neves I, Sales JS et al. (2003) Induction of apoptosis in monocytes by Mycobacterium leprae in vitro: a possible role for tumour necrosis factor-alpha. Immunology 109:156–164
Rambukkana A, Zanazzi G, Tapinos N, Salzer JL (2002) Contact-dependent demyelination by Mycobacterium leprae in the absence of immune cells. Science 296:927–931
Oliveira RB, Ochoa MT, Sieling PA et al. (2003) Expression of Toll-like receptor 2 on human Schwann cells: a mechanism of nerve damage in leprosy. Infect Immun 71:1427–1433
Filion MC, Lepicier P, Morales A, Phillips NC (1999) Mycobacterium phlei cell wall complex directly induces apoptosis in human bladder cancer cells. Br J Cancer 79:229–235
Filion MC, Phillips NC (2001) Therapeutic potential of mycobacterial cell wall-DNA complexes. Expert Opin Investig Drugs 10:2157–2165
Reader S, Menard S, Filion B, Filion MC, Phillips NC (2001) Pro-apoptotic and immunomodulatory activity of a mycobacterial cell wall-DNA complex towards LNCaP prostate cancer cells. Prostate 49:155–165
George KM, Pascopella L, Welty DM, Small PL (2000) A Mycobacterium ulcerans toxin, mycolactone, causes apoptosis in guinea pig ulcers and tissue culture cells. Infect Immun 68:877–883
Trebichavsky I, Barot-Ciorbaru R, Charley B, Splichal I (1993) Induction of inflammatory cytokines by Nocardia fractions. Folia Biol (Praha) 39:243–249
Tam S, Barry DP, Beaman L, Beaman BL (2002) Neuroinvasive Nocardia asteroides GUH-2 induces apoptosis in the substantia nigra in vivo and dopaminergic cells in vitro. Exp Neurol 177:453–460
Camp DM, Loeffler DA, Razoky BA et al. (2003) Nocardia asteroides culture filtrates cause dopamine depletion and cytotoxicity in PC12 cells. Neurochem Res 28:1359–1367
Loeffler DA, Camp DM, Qu S, Beaman BL, LeWitt PA (2004) Characterization of dopamine-depleting activity of Nocardia asteroides strain GUH-2 culture filtrate on PC12 cells. Microb Pathog 37:73–85
Sinkora M, Mandel L, Sinkora J et al. (1997) Bacterial immunomodulators affect programmed cell death of mouse spleen lymphocytes. Ann N Y Acad Sci 815:492–495
Luhrmann A, Mauder N, Sydor T et al. (2004) Necrotic death of Rhodococcus equi-infected macrophages is regulated by virulence-associated plasmids. Infect Immun 72:853–862
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barry, D.P., Beaman, B.L. Modulation of eukaryotic cell apoptosis by members of the bacterial order Actinomycetales. Apoptosis 11, 1695–1707 (2006). https://doi.org/10.1007/s10495-006-9236-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-006-9236-3