Abstract
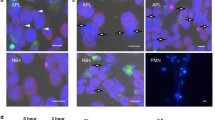
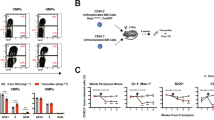
Peptidylarginine deiminases (PADIs) convert peptidylarginine into citrulline via posttranslational modification. One member of the family, PADI4, plays an important role in immune cell differentiation and cell death. To elucidate the participation of PADI4 in haematopoietic cell death, we examine whether inducible overexpression of PADI4 enhances the apoptotic cell death. PADI4 reduced the viability in a dose- and time-dependent manner of human leukemia HL-60 cells and human acute T leukemia Jurkat cells. The apoptosis-inducing activities were determined by nuclear condensation, DNA fragmentation, sub-G1 appearance, loss of mitochondrial membrane potential (Δψm), release of mitochondrial cytochrome c into cytoplasm and proteolytic activation of caspase 9 and 3. Following PADI4 overexpression, cells arrest in G1 phase significantly before their entrance into apoptotic cell death. PADI4 increases tumor suppressor p53 and its downstream p21 to control cell cycle. In the detections of protein expression and kinase activity, all protein levels of cyclin-dependent kinases (CDKs) and cyclins are not reduced except cyclin D, however, CDK2 (G1 entry S phase) and CDK1 (G2 entry M phase) enzyme activities are inhibited by conditionally inducible PADI4. p53 also expands its other downstream Bax to induce cytochrome c release from mitochondria. According to these data, we suggest that PADI4 induces apoptosis mainly through cell cycle arrest and mitochondria-mediated pathway. Furthermore, p53 features in PADI4-induced apoptosis by increasing intracellular p21 to control cell cycle and by Bax accumulation to decline Bcl-2 function, destroy Δψm, release cytochrome c to cytoplasm and activate the caspase cascade.
Similar content being viewed by others
Abbreviations
- PADIs:
-
peptidylarginine deiminases
- Δψm:
-
mitochondrial membrane potential
- CDKs:
-
cyclin-dependent kinases.
References
Guerrin M, Ishigami A, Mechin MC, et al. cDNA cloning, gene organization and expression analysis of human peptidylarginine deiminase type I. Biochem J 2003; 370: 167–174.
Ishigami A, Ohsawa T, Asaga H, Akiyama K, Kuramoto M, Maruyama N. Human peptidylarginine deiminase type II: Molecular cloning, gene organization, and expression in human skin. Arch Biochem Biophys 2002; 407: 25–31.
Kanno T, Kawada A, Yamanouchi J, et al. Human peptidylarginine deiminase type III: Molecular cloning and nucleotide sequence of the cDNA, properties of the recombinant enzyme, and immunohistochemical localization in human skin. J Invest Dermatol 2000; 115: 813–823.
Nakashima K, Hagiwara T, Ishigami A, et al. Molecular characterization of peptidylarginine deiminase in HL-60 cells induced by retinoic acid and 1alpha, 25-dihydroxyvitamin D(3). J Biol Chem 1999; 274: 27786–27792.
Chavanas S, Mechin MC, Takahara H, et al. Comparative analysis of the mouse and human peptidylarginine deiminase gene clusters reveals highly conserved non-coding segments and a new human gene, PADI6. Gene 2004; 330: 19–27.
Nijenhuis S, Zendman AJ, Vossenaar ER, Pruijn GJ, van Venrooij WJ. Autoantibodies to citrullinated proteins in rheumatoid arthritis: clinical performance and biochemical aspects of an RA-specific marker. Clin Chim Acta 2004; 350:17–34.
Chang X, Yamada R, Suzuki A, et al. Localization of peptidylarginine deiminase 4 (PADI4) and citrullinated protein in synovial tissue of rheumatoid arthritis. Rheumatology (Oxford) 2005; 44: 40–50.
Senshu T, Akiyama K, Ishigami A, Nomura K. Studies on specificity of peptidylarginine deiminase reactions using an immunochemical probe that recognizes an enzymatically deiminated partial sequence of mouse keratin K1. J Dermatol Sci 1999; 21: 113–126.
Ishida-Yamamoto A, Senshu T, Eady RA, et al. Sequential reorganization of cornified cell keratin filaments involving filaggrin-mediated compaction and keratin 1 deimination. J Invest Dermatol 2002; 118: 282–287.
Tarcsa E, Marekov LN, Mei G, Melino G, Lee SC, Steinert PM. Protein unfolding by peptidylarginine deiminase. Substrate specificity and structural relationships of the natural substrates trichohyalin and filaggrin. J Biol Chem 1996; 271: 30709–30716.
Vossenaar ER, Despres N, Lapointe E, et al. Rheumatoid arthritis specific anti-Sa antibodies target citrullinated vimentin. Arthritis Res Ther 2004; 6: R142–R150.
Moscarello MA, Wood DD, Ackerley C, Boulias C. Myelin in multiple sclerosis is developmentally immature. J Clin Invest 1994; 94: 146–154.
Wang Y, Wysocka J, Sayegh J, et al. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science 2004; 306: 279–283.
Cuthbert GL, Daujat S, Snowden AW, et al. Histone deimination antagonizes arginine methylation. Cell 2004; 118:545–553.
Vossenaar ER, Nijenhuis S, Helsen MM, et al. Citrullination of synovial proteins in murine models of rheumatoid arthritis. Arthritis Rheum 2003; 48: 2489–2500.
Caponi L, Petit-Teixeira E, Sebbag M, et al. A family based study shows no association between rheumatoid arthritis and the PADI4 gene in a white French population. Ann Rheum Dis 2005; 64: 587–593.
Masson-Bessiere C, Sebbag M, Girbal-Neuhauser E, et al. The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the alpha- and beta-chains of fibrin. J Immunol 2001; 166: 4177–4184.
Suzuki A, Yamada R, Ohtake-Yamanaka M, Okazaki Y, Sawada T, Yamamoto K. Anti-citrullinated collagen type I antibody is a target of autoimmunity in rheumatoid arthritis. Biochem Biophys Res Commun 2005; 333: 418–426.
Wood DD, Bilbao JM, O'Connors P, Moscarello MA. Acute multiple sclerosis (Marburg type) is associated with developmentally immature myelin basic protein. Ann Neurol 1996; 40: 18–24.
Wright PW, Bolling LC, Calvert ME, et al. ePAD, an oocyte and early embryo-abundant peptidylarginine deiminase-like protein that localizes to egg cytoplasmic sheets. Dev Biol 2003; 256: 73–88.
Asaga H, Yamada M, Senshu T. Selective deimination of vimentin in calcium ionophore-induced apoptosis of mouse peritoneal macrophages. Biochem Biophys Res Commun 1998; 243: 641–646.
Mizoguchi M, Manabe M, Kawamura Y, et al. Deimination of 70-kD nuclear protein during epidermal apoptotic events in vitro. J Histochem Cytochem 1998; 46: 1303–1309.
Senshu T, Akiyama K, Nagata S, Watanabe K, Hikichi K. Peptidylarginine deiminase in rat pituitary: Sex difference, estrous cycle-related changes, and estrogen dependence. Endocrinology 1989; 124: 2666–2670.
Okuda M. The role of nucleophosmin in centrosome duplication. Oncogene 2002; 21: 6170–6174.
Hingorani K, Szebeni A, Olson MO. Mapping the functional domains of nucleolar protein B23. J Biol Chem 2000; 275: 24451–24457.
Takemura M, Ohoka F, Perpelescu M, et al. Phosphorylation-dependent migration of retinoblastoma protein into the nucleolus triggered by binding to nucleophosmin/B23. Exp Cell Res 2002; 276: 233–241.
Hagiwara T, Nakashima K, Hirano H, Senshu T, Yamada M. Deimination of arginine residues in nucleophosmin/B23 and histones in HL-60 granulocytes. Biochem Biophys Res Commun 2002; 290: 979–983.
Hagiwara T, Hidaka Y, Yamada M. Deimination of histone H2A and H4 at arginine 3 in HL-60 granulocytes. Biochemistry 2005; 44: 5827–5834.
Liu GY, Hung YC, Hsu PC, et al. Ornithine decarboxylase prevents tumor necrosis factor alpha-induced apoptosis by decreasing intracellular reactive oxygen species. Apoptosis 2005; 10: 569–581.
Boyde TR, Rahmatullah M. Optimization of conditions for the colorimetric determination of citrulline, using diacetyl monoxime. Anal. Biochem. 1980; 107: 424–431.
Huang HH, Hsu PC, Hung YC et al. Ornithine decarboxylase prevents methotrexate-induced apoptosis by reducing intracellular reactive oxygen species production. Apoptosis 2005; 10: 895–907.
Lum JJ, Bauer DE, Kong M, et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell 2005; 120: 237–248.
Levine B. Eating oneself and uninvited guests: Autophagy-related pathways in cellular defense. Cell 2005; 120: 159–162.
Vossenaar ER, Radstake TR, van der Heijden A, et al. Expression and activity of citrullinating peptidylarginine deiminase enzymes in monocytes and macrophages. Ann Rheum Dis 2004; 63: 373–381.
Inagaki M, Takahara H, Nishi Y, Sugawara K, Sato C. Ca2+-dependent deimination-induced disassembly of intermediate filaments involves specific modification of the amino-terminal head domain. J Biol Chem 1989; 264: 18119–18127.
Arita K, Hashimoto H, Shimizu T, Nakashima K, Yamada M, Sato M. Structural basis for Ca(2+)-induced activation of human PAD4. Nat Struct Mol Biol 2004; 11: 777–783.
Nakashima K, Hagiwara T, Yamada M. Nuclear localization of peptidylarginine deiminase V and histone deimination in granulocytes. J Biol Chem 2002; 277: 49562–49568.
Kastan MB, Zhan Q, el-Deiry WS, et al. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell 1992; 71: 587–597.
Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature 1993; 362: 847–849.
Woodworth CD, Wang H, Simpson S, Alvarez-Salas LM, Notario V. Overexpression of wild-type p53 alters growth and differentiation of normal human keratinocytes but not human papillomavirus-expressing cell lines. Cell Growth Differ 1993; 4: 367–376.
Weinberg WC, Azzoli CG, Chapman K, Levine AJ, Yuspa SH. p53-mediated transcriptional activity increases in differentiating epidermal keratinocytes in association with decreased p53 protein. Oncogene 1995; 10: 2271–2279.
Hall PA, McKee PH, Menage HD, Dover R, Lane DP. High levels of p53 protein in UV-irradiated normal human skin. Oncogene 1993; 8: 203–207.
Berg RJ, van Kranen HJ, Rebel HG, et al. Early p53 alterations in mouse skin carcinogenesis by UVB radiation: immunohistochemical detection of mutant p53 protein in clusters of preneoplastic epidermal cells. Proc Natl Acad Sci USA 1996; 93: 274–278.
Li G, Mitchell DL, Ho VC, Reed JC, Tron VA. Decreased DNA repair but normal apoptosis in ultraviolet-irradiated skin of p53-transgenic mice. Am J Pathol 1996; 148: 1113–1123.
Appella E, Anderson CW. Signaling to p53: Breaking the posttranslational modification code. Pathol Biol (Paris) 2000; 48: 227–245.
Munoz-Alonso MJ, Acosta JC, Richard C, Delgado MD, Sedivy J, Leon J. p21Cip1 and p27Kip1 induce distinct cell cycle effects and differentiation programs in myeloid leukemia cells. J Biol Chem 2005; 280: 18120–18129.
Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 1999; 13: 1501–1512.
Gilmour SK, Birchler M, Smith MK, Rayca K, Mostochuk J. Effect of elevated levels of ornithine decarboxylase on cell cycle progression in skin. Cell Growth Differ 1999; 10: 739–748.
Norbury CJ, Zhivotovsky B. DNA damage-induced apoptosis. Oncogene 2004; 23: 2797–2808.
Chao DT, Korsmeyer SJ. BCL-2 family: Regulators of cell death. Annu Rev Immunol 1998; 16: 395–419.
Schuler M, Green DR. Transcription, apoptosis and p53: catch-22. Trends Genet 2005; 21: 182–187.
Chipuk JE, Kuwana T, Bouchier-Hayes L, et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 2004; 303: 1010–1014.
Byun Y, Chen F, Chang R, Trivedi M, Green KJ, Cryns VL. Caspase cleavage of vimentin disrupts intermediate filaments and promotes apoptosis. Cell Death Differ 2001; 8: 443–450.
Germain D, Russell A, Thompson A, Hendley J. Ubiquitination of free cyclin D1 is independent of phosphorylation on threonine 286. J Biol Chem 2000; 275: 12074–12079.
Newman RM, Mobascher A, Mangold U, et al. Antizyme targets cyclin D1 for degradation. A novel mechanism for cell growth repression. J Biol Chem 2004; 279: 41504–41511.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, GY., Liao, YF., Chang, WH. et al. Overexpression of peptidylarginine deiminase IV features in apoptosis of haematopoietic cells. Apoptosis 11, 183–196 (2006). https://doi.org/10.1007/s10495-006-3715-4
Issue Date:
DOI: https://doi.org/10.1007/s10495-006-3715-4