Abstract
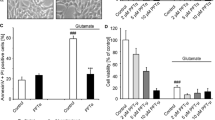
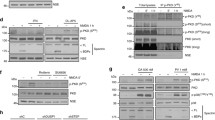
The highly frequent mutation of phosphatase and tensin homologue deleted on chromosome 10 (PTEN) in various cancers has attracted much attention to study its role in tumorigenesis. As an important tumor suppressor, the pro-apoptotic function of PTEN has been linked to its capacity antagonizing the PI3K/Akt signaling pathway. However, less data are available concerning its role in neurodegeneration in which apoptotic processes are also involved. In the present study, we attempted to study the role and the underlying mechanism of PTEN in neuronal apoptosis. Using primary rat hippocampal cultures, staurosporine (STS, 100 nM) induced a time-dependent apoptosis, accompanied by a marked production of reactive oxygen species (ROS), release of cytochrome c and activation of caspase 9 and 3. However, the expression of PTEN, and the levels of phospho-PTEN and phospho-Akt were not changed at all time points tested (0.5–24 h) after STS stimulation, suggesting that the protein level as well as the phosphorylation status of PTEN were not related to the procession of apoptosis. Interestingly, immunostaining revealed a punctate intracellular distribution of PTEN from 2 to 8 h after adding STS. Double labeling and Western blotting of mitochondrial fraction demonstrated a mitochondrial location and accumulation of PTEN, respectively, after challenging with STS. Furthermore, we provide evidence for the first time that PTEN was associated with Bax in the absence and the presence of STS. Of note, the STS-induced marked increase in the cellular ROS level, release of cytochrome c and activation of caspase 3 were inhibited in cultured hippocampal cells when PTEN was knocked down by a specific antisense. Moreover, knockdown of PTEN significantly protected hippocampal cells from apoptotic damage. These findings demonstrated that PTEN is a crucial mediator of mitochondria-dependent apoptosis, and thus could become a molecular target for interfering with neurodegenerative diseases.
Similar content being viewed by others
References
Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 1997; 275: 1943–1947.
Steck PA, Pershouse MA, Jasser SA, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet 1997; 15: 356–362.
Myers MP, Stolarov JP, Eng C, et al. P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc Natl Acad Sci U S A 1997; 94: 9052–9057.
Gu J, Tamura M, Yamada KM. Tumor suppressor PTEN inhibits integrin- and growth factor-mediated mitogen-activated protein (MAP) kinase signaling pathways. J Cell Biol 1998; 143: 1375–1383.
Tamura M, Gu J, Danen EH, Takino T, Miyamoto S, Yamada KM. PTEN interactions with focal adhesion kinase and suppression of the extracellular matrix-dependent phosphatidylinositol 3-kinase/Akt cell survival pathway. J Biol Chem 1999; 274: 20693–20703.
Besson A, Robbins SM, Yong VW. PTEN/MMAC1/TEP1 in signal transduction and tumorigenesis. Eur J Biochem 1999; 263: 605–611.
Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 1998; 273: 13375–13378.
Leslie NR, Downes CP. PTEN: The down side of PI 3-kinase signalling. Cell Signal 2002; 14: 285–295.
Downward J. PI 3-kinase, Akt and cell survival. Semin Cell Dev Biol 2004; 15: 177–182.
Vazquez F, Sellers WR. The PTEN tumor suppressor protein: An antagonist of phosphoinositide 3-kinase signaling. Biochim Biophys Acta 2000; 1470: M21–35.
Steelman LS, Bertrand FE, McCubrey JA. The complexity of PTEN: mutation, marker and potential target for therapeutic intervention. Expert Opin Ther Targets 2004; 8: 537–550.
Li L, Liu F, Salmonsen RA, et al. PTEN in neural precursor cells: Regulation of migration, apoptosis, and proliferation. Mol Cell Neurosci 2002; 20: 21–29.
Li L, Liu F, Ross AH. PTEN regulation of neural development and CNS stem cells. J Cell Biochem 2003; 88: 24–28.
Lachyankar MB, Sultana N, Schonhoff CM, et al. A role for nuclear PTEN in neuronal differentiation. J Neurosci 2000; 20: 1404–1413.
Perandones C, Costanzo RV, Kowaljow V, Pivetta OH, Carminatti H, Radrizzani M. Correlation between synaptogenesis and the PTEN phosphatase expression in dendrites during postnatal brain development. Brain Res Mol Brain Res 2004; 128: 8–19.
Backman SA, Stambolic V, Suzuki A, et al. Deletion of Pten in mouse brain causes seizures, ataxia and defects in soma size resembling Lhermitte-Duclos disease. Nat Genet 2001; 29: 396–403.
Kwon CH, Zhu X, Zhang J, et al. Pten regulates neuronal soma size: a mouse model of Lhermitte-Duclos disease. Nat Genet 2001; 29: 404–411.
Kwon CH, Zhu X, Zhang J, Baker SJ. mTor is required for hypertrophy of Pten-deficient neuronal soma in vivo. Proc Natl Acad Sci USA 2003; 100: 12923–12928.
Gary DS, Mattson MP. PTEN regulates Akt kinase activity in hippocampal neurons and increases their sensitivity to glutamate and apoptosis. Neuromolecular Med 2002; 2: 261–269.
Lee JH, Kim KY, Lee YK, et al. Cilostazol prevents focal cerebral ischemic injury by enhancing casein kinase 2 phosphorylation and suppression of phosphatase and tensin homolog deleted from chromosome 10 phosphorylation in rats. J Pharmacol Exp Ther 2004; 308: 896–903.
Ning K, Pei L, Liao M, et al. Dual neuroprotective signaling mediated by downregulating two distinct phosphatase activities of PTEN. J Neurosci 2004; 24: 4052–4060.
Kyrylenko S, Roschier M, Korhonen P, Salminen A. Regulation of PTEN expression in neuronal apoptosis. Brain Res Mol Brain Res 1999; 73: 198–202.
Sternberger M, Schmiedeknecht A, Kretschmer A, et al. GeneBlocs are powerful tools to study and delineate signal transduction processes that regulate cell growth and transformation. Antisense Nucleic Acid Drug Dev 2002; 12: 131–143.
Zhu Y, Ahlemeyer B, Bauerbach E, Krieglstein J. TGF-ß1 inhibits caspase-3 activation and neuronal apoptosis in rat hippocampal cultures. Neurochem Int 2001; 38: 227–235.
Bindokas VP, Jordan J, Lee CC, Miller RJ. Superoxide production in rat hippocampal neurons: Selective imaging with hydroethidine. J Neurosci 1996; 16: 1324–1336.
Ahlemeyer B, Bauerbach E, Plath M, et al. Retinoic acid reduces apoptosis and oxidative stress by preservation of SOD protein level. Free Radic Biol Med 2001; 30: 1067–1077.
Kretschmer A, Moepert K, Dames S, Sternberger M, Kaufmann J, Klippel A. Differential regulation of TGF-beta signaling through Smad2, Smad3 and Smad4. Oncogene 2003; 22: 6748–6763.
Torres J, Pulido R. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. Implications for PTEN stability to proteasome-mediated degradation. J Biol Chem 2001; 276: 993–998.
Birle D, Bottini N, Williams S, et al. Negative feedback regulation of the tumor suppressor PTEN by phosphoinositide-induced serine phosphorylation. J Immunol 2002; 169: 286–291.
Miller SJ, Lou DY, Seldin DC, Lane WS, Neel BG. Direct identification of PTEN phosphorylation sites. FEBS Lett 2002; 528: 145–153.
Das S, Dixon JE, Cho W. Membrane-binding and activation mechanism of PTEN. Proc Natl Acad Sci USA 2003; 100: 7491–7496.
Frese KK, Lee SS, Thomas DL, et al. Selective PDZ protein-dependent stimulation of phosphatidylinositol 3-kinase by the adenovirus E4-ORF1 oncoprotein. Oncogene 2003; 22: 710–721.
Griffin RJ, Moloney A, Kelliher M, et al. Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer's disease pathology. J Neurochem 2005; 93: 105–117.
Yuan J, Lipinski M, Degterev A. Diversity in the mechanisms of neuronal cell death. Neuron 2003; 40: 401–13.
Valentijn AJ, Metcalfe AD, Kott J, Streuli CH, Gilmore AP. Spatial and temporal changes in Bax subcellular localization during anoikis. J Cell Biol 2003; 162: 599–612.
Green DR, Reed JC. Mitochondria and apoptosis. Science 1998; 281: 1309–1312.
Stambolic V, MacPherson D, Sas D, et al. Regulation of PTEN transcription by p53. Mol Cell 2001; 8: 317–325.
Freeman DJ, Li AG, Wei G, et al. PTEN tumor suppressor regulates p53 protein levels and activity through phosphatase-dependent and -independent mechanisms. Cancer Cell 2003; 3: 117–130.
Krohn AJ, Wahlbrink T, Prehn HM. Mitochondrial depolarization is not required for neuronal apoptosis. J Neurosci 1999; 19: 7394–7404.
Vander Heiden MG, Chandel NS, Williamson EK, et al. Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell 1997; 91: 627–637.
Reed JC. Cytochrome c: can't live with it–can't live without it. Cell 1997; 91: 559–562.
Polster BM, Fiskum G. Mitochondrial mechanisms of neural cell apoptosis. J Neurochem 2004; 90: 1281–1289.
Kirkland RA, Franklin JL. Bax, reactive oxygen, and cytochrome c release in neuronal apoptosis. Antioxid Redox Signal 2003; 5: 589–96.
Shimizu T, Numata T, Okada Y. A role of reactive oxygen species in apoptotic activation of volume-sensitive Cl(-) channel. Proc Natl Acad Sci U S A 2004; 101: 6770–6773.
Li D, Ueta E, Kimura T, Yamamoto T, Osaki T. Reactive oxygen species (ROS) control the expression of Bcl-2 family proteins by regulating their phosphorylation and ubiquitination. Cancer Sci 2004; 95: 644–650.
Buccellato LJ, Tso M, Akinci OI, Chandel NS, Budinger GR. Reactive oxygen species are required for hyperoxia-induced Bax activation and cell death in alveolar epithelial cells. J Biol Chem 2004; 279: 6753–6760.
Kirkland RA, Windelborn JA, Kasprzak JM, Franklin JL. A Bax-induced pro-oxidant state is critical for cytochrome c release during programmed neuronal death. J Neurosci 2002; 22: 6480–90.
Ostrakhovitch EA, Cherian MG. Role of p53 and reactive oxygen species in apoptotic response to copper and zinc in epithelial breast cancer cells. Apoptosis 2005; 10: 111–121.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, Y., Hoell, P., Ahlemeyer, B. et al. PTEN: A crucial mediator of mitochondria-dependent apoptosis. Apoptosis 11, 197–207 (2006). https://doi.org/10.1007/s10495-006-3714-5
Issue Date:
DOI: https://doi.org/10.1007/s10495-006-3714-5