Abstract
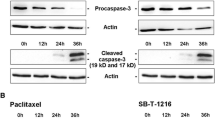
Aberrant apoptosis has been associated with the development and therapeutic resistance of cancer. Recent studies suggest that caspase deficiency/downregulation is frequently detected in different cancers. We have previously shown that caspase-3 reconstitution significantly sensitized MCF-7 cells to doxorubicin and etoposide. In contrast to the well established role of caspase-3 as an effector caspase, the focus of this study is to delineate caspase-3 induced feedback activation of the apical caspases-2, -8, -9 and -10A in doxorubicin and TNF-α induced apoptosis. Using cell-free systems we show that caspases-9 and 2 are the most sensitive, caspase-8 is less sensitive and caspase-10A is the least sensitive to caspase-3 mediated-cleavage. When apoptosis is induced by doxorubicin or TNF-α in an intact cell model, cleavage of caspases-8 and -9, but not caspase-2, was markedly enhanced by caspase-3. Caspase-3 mediated-feedback and activation of caspase-8 and -9 in MCF-7/C3 cells is further supported by an increase in the cleavage of caspase-8 and 9 substrates and cytochrome c release. These data indicate that, in addition to its function as an effector caspase, caspase-3 plays an important role in maximizing the activation of apical caspases and crosstalk between the two major apoptotic pathways. The significant impact of caspase-3 on both effector and apical caspases suggests that modulation of caspase-3 activity would be a useful approach to overcome drug resistance in clinical oncology.
Similar content being viewed by others
Abbreviations
- DISC:
-
death-inducing signaling complex
- CHX:
-
cycloheximide
- TnT:
-
in vitro transcription and translation
- DEVD-CHO:
-
Ac-Asp-Glu-Val-Asp-CHO
- YVAD-CHO:
-
Ac-Tyr-Val-Ala-Asp-CHO
- Z-IETD-AMC:
-
Z-Ile-Glu-Thr-Asp-AMC
- Ac-LEHD-AMC:
-
Ac-Leu-Glu-His-Asp-AMC
- PARP:
-
poly(ADP-ribose)polymerases
References
Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J (1996) Human ICE/CED-3 protease nomenclature. Cell 87:171
Thornberry NA, Lazebnik Y (1998) Caspases: enemies within. Science 281:1312–1316
Salvesen GS, Dixit VM (1997) Caspases: intracellular signaling by proteolysis. Cell 91:443–446
Denault JB, Salvesen GS (2002) Caspases: keys in the ignition of cell death. Chem Rev 102:4489–4500
Froelich CJ, Dixit VM, Yang X (1998) Lymphocyte granule-mediated apoptosis: matters of viral mimicry and deadly proteases. Immunol Today 19:30–36
Hengartner MO (2000) The biochemistry of apoptosis. Nature 407:770–776
Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, Peter ME (1995) Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. Embo J 14:5579–5588
Salvesen GS, Dixit VM (1999) Caspase activation: the induced-proximity model. Proc Natl Acad Sci USA 96:10964–10967
Wang X (2001) The expanding role of mitochondria in apoptosis. Genes Dev 15:2922–2933
Green DR, Kroemer G (2004) The pathophysiology of mitochondrial cell death. Science 305:626–629
Zou H, Li Y, Liu X, Wang X (1999) An APAF-1. cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem 274:11549–11556
Shi Y (2002) Apoptosome: the cellular engine for the activation of caspase-9. Structure (Camb) 10:285–288
Boatright KM, Salvesen GS (2003) Mechanisms of caspase activation. Curr Opin Cell Biol 15:725–731
Fischer U, Janicke RU, Schulze-Osthoff K (2003) Many cuts to ruin: a comprehensive update of caspase substrates. Cell Death Differ 10:76–100
Porter AG, Janicke RU (1999) Emerging roles of caspase-3 in apoptosis. Cell Death Differ 6: 99–104
Kuida K, Zheng TS, Na S, Kuan C, Yang D, Karasuyama H, Rakic P, Flavell RA (1996) Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature 384:368–372
Janicke RU, Sprengart ML, Wati MR, Porter AG (1998) Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem 273:9357–9360
Yang XH, Sladek TL, Liu X, Butler BR, Froelich CJ, Thor AD (2001) Reconstitution of caspase 3 sensitizes MCF-7 breast cancer cells to doxorubicin- and etoposide-induced apoptosis. Cancer Res 61:348–354
Yang XH, Edgerton S, Thor AD (2005) Reconstitution of caspase-3 sensitizes MCF-7 breast cancer cells to radiation therapy. Int J Oncol 26:1675–1680
Yang X, Stennicke HR, Wang B, Green DR, Janicke RU, Srinivasan A, Seth P, Salvesen GS, Froelich CJ (1998) Granzyme B mimics apical caspases. Description of a unified pathway for trans-activation of executioner caspase-3 and -7. J Biol Chem 273:34278–34283
Devarajan E, Sahin AA, Chen JS, Krishnamurthy RR, Aggarwal N, Brun AM, Sapino A, Zhang F, Sharma D, Yang XH, Tora AD, Mehta K (2002) Down-regulation of caspase 3 in breast cancer: a possible mechanism for chemoresistance. Oncogene 21:8843–8851
Fujita E, Egashira J, Urase K, Kuida K, Momoi T (2001) Caspase-9 processing by caspase-3 via a feedback amplification loop in vivo. Cell Death Differ 8:335–344
Blanc C, Deveraux QL, Krajewski S, Janicke RU, Porter AG, Reed JC, Jaggi R, Marti A (2000) Caspase-3 is essential for procaspase-9 processing and cisplatin-induced apoptosis of MCF-7 breast cancer cells. Cancer Res 60:4386–4390
Stennicke HR, Jurgensmeier JM, Shin H, Deveraux Q, Wolf BB, Yang X, Zhou Q, Ellerby HM, Ellerby LM, Bredesen D, Green DR, Reed JC, Froelich CJ, Salvesen GS (1998) Pro-caspase-3 is a major physiologic target of caspase-8. J Biol Chem 273:27084–27090
Talanian RV, Yang X, Turbov J, Seth P, Ghayur T, Casiano CA, Orth K, Froelich CJ (1997) Granule-mediated killing: pathways for granzyme B-initiated apoptosis. J Exp Med 186:1323–1331
Yang XH, Sladek TL (1995) Overexpression of the E2F-1 transcription factor gene mediates cell transformation. Gene Expr 4:195–204
Bossy-Wetzel E, Green DR (2000) Assays for cytochrome c release from mitochondria during apoptosis. Methods Enzymol 322:235–242
Srinivasula SM, Ahmad M, Fernandes-Alnemri T, Alnemri ES (1998) Autoactivation of procaspase-9 by Apaf-1-mediated oligomerization. Mol Cell 1:949–957
Johnson CR, Jarvis WD (2004) Caspase-9 regulation: an update. Apoptosis 9:423–427
Li H, Bergeron L, Cryns V, Pasternack MS, Zhu H, Shi L, Greenberg A, Yuan J (1997) Activation of caspase-2 in apoptosis. J Biol Chem 272:21010–21017
Luo X, Budihardjo I, Zou H, Slaughter C, Wang X (1998) Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94:481–490
Slee EA, Keogh SA, Martin SJ (2000) Cleavage of BID during cytotoxic drug and UV radiation-induced apoptosis occurs downstream of the point of Bcl-2 action and is catalysed by caspase-3: a potential feedback loop for amplification of apoptosis-associated mitochondrial cytochrome c release. Cell Death Differ 7:556–565
Deveraux QL, Roy N, Stennicke HR, Van Arsdale T, Zhou Q, Srinivasula SM, Alnemri ES, Salvesen GS, Reed JC (1998) IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. Embo J 17:2215–2223
Pan G, O'Rourke K, Dixit VM (1998) Caspase-9, Bcl-XL, and Apaf-1 form a ternary complex. J Biol Chem 273:5841–5845
Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC (1998) Regulation of cell death protease caspase-9 by phosphorylation. Science 282:1318–1321
Allan LA, Morrice N, Brady S, Magee G, Pathak S, Clarke PR (2003) Inhibition of caspase-9 through phosphorylation at Thr 125 by ERK MAPK. Nat Cell Biol 5:647–654
Rodriguez J, Lazebnik Y (1999) Caspase-9 and APAF-1 form an active holoenzyme. Genes Dev 13:3179–3184
Shi Y (2004) Caspase activation: revisiting the induced proximity model. Cell 117:855–858
Medema JP, Scaffidi C, Kischkel FC, Shevchenko A, Mann M, Krammer PH, Peter ME (1997) FLICE is activated by association with the CD95 death-inducing signaling complex (DISC). Embo J 16:2794–2804
Li H, Zhu H, Xu CJ, Yuan J (1998) Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491–501
Cowling V, Downward J (2002) Caspase-6 is the direct activator of caspase-8 in the cytochrome c-induced apoptosis pathway: absolute requirement for removal of caspase-6 prodomain. Cell Death Differ 9:1046–1056
Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, Wang HG, Reed JC, Nicholson DW, Alnemri ES, Green DR, Martin SJ (1999) Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol 144:281–292
Paroni G, Henderson C, Schneider C, Brancolini C (2001) Caspase-2-induced apoptosis is dependent on caspase-9, but its processing during UV- or tumor necrosis factor-dependent cell death requires caspase-3. J Biol Chem 276:21907–21915
Cheng EH, Kirsch DG, Clem RJ, Ravi R, Kastan MB, Bedi A, Ueno K, Hardwick JM (1997) Conversion of Bcl-2 to a Bax-like death effector by caspases. Science 278:1966–1968
Lakhani SA, Masud A, Kuida K, Porter, Jr GA, Booth CJ, Mehal WZ, Inayat I, Flavell RA (2006) Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science 311:847–851
Author information
Authors and Affiliations
Corresponding author
Additional information
XiaoHe Yang: This work was supported in part by the Career Development Award DAMD17-99-1-9180 from Department of Defense to X.H.Y.
Rights and permissions
About this article
Cite this article
Yang, S., Thor, A.D., Edgerton, S. et al. Caspase-3 mediated feedback activation of apical caspases in doxorubicin and TNF-α induced apoptosis. Apoptosis 11, 1987–1997 (2006). https://doi.org/10.1007/s10495-006-0084-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-006-0084-y