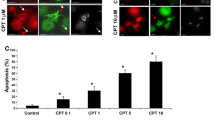
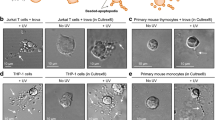
Abstract
It has recently been shown that the microtubule cytoskeleton is reformed during the execution phase of apoptosis. We demonstrate that this microtubule reformation occurs in many cell types and under different apoptotic stimuli. We confirm that the apoptotic microtubule network possesses a novel organization, whose nucleation appears independent of conventional γ-tubulin ring complex containing structures. Our analysis suggests that microtubules are closely associated with the plasma membrane, forming a cortical ring or cellular “cocoon”. Concomitantly other components of the cytoskeleton, such as actin and cytokeratins disassemble. We found that colchicine-mediated disruption of apoptotic microtubule network results in enhanced plasma membrane permeability and secondary necrosis, suggesting that the reformation of a microtubule cytoskeleton plays an important role in preserving plasma membrane integrity during apoptosis. Significantly, cells induced to enter apoptosis in the presence of the pan-caspase inhibitor z-VAD, nevertheless form microtubule-like structures suggesting that microtubule formation is not dependent on caspase activation. In contrast we found that treatment with EGTA-AM, an intracellular calcium chelator, prevents apoptotic microtubule network formation, suggesting that intracellular calcium may play an essential role in the microtubule reformation. We propose that apoptotic microtubule network is required to maintain plasma membrane integrity during the execution phase of apoptosis.










Similar content being viewed by others
Abbreviations
- AMN:
-
apoptotic microtubule network
- CPT:
-
camptothecin
- COL:
-
colchicine
- CYTO:
-
cytochalasin
- EGTA-AM:
-
Ethyleneglycol-bis(b-aminoethyl)-N,N,N′,N′- tetraacetoxymethyl Ester
- LDH:
-
lactic dehydrogenase
References
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26(4):239–257
Savill J, Dransfield I, Gregory C, Haslett C (2002) A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol 2(12):965–975
Wilson MR (1998) Apoptosis: unmasking the executioner. Cell Death Differ 5(8):646–652
Afford S, Randhawa S (2000) Apoptosis. Mol Pathol 53(2):55–63
Mills JC, Stone NL, Pittman RN (1999) Extranuclear apoptosis. The role of the cytoplasm in the execution phase. J Cell Biol 146(4):703–708
Pittman S, Geyp M, Fraser M, Ellem K, Peaston A, Ireland C (1997) Multiple centrosomal microtubule organising centres and increased microtubule stability are early features of VP-16-induced apoptosis in CCRF-CEM cells. Leuk Res 21(6):491–499
Pittman SM, Strickland D, Ireland CM (1994) Polymerization of tubulin in apoptotic cells is not cell cycle dependent. Exp Cell Res 215(2):263–272
Moss DK, Lane JD (2006) Microtubules: forgotten players in the apoptotic execution phase. Trends Cell Biol 16(7):330–338
Moss DK, Betin VM, Malesinski SD, Lane JD (2006) A novel role for microtubules in apoptotic chromatin dynamics and cellular fragmentation. J Cell Sci 119(Pt 11):2362–2374
Brea-Calvo G, Rodriguez-Hernandez A, Fernandez-Ayala DJ, Navas P, Sanchez-Alcazar JA (2006) Chemotherapy induces an increase in coenzyme Q10 levels in cancer cell lines. Free Radic Biol Med 40(8):1293–1302
Sanchez-Alcazar JA, Bradbury DA, Brea-Calvo G, Navas P, Knox AJ (2003) Camptothecin-induced apoptosis in non-small cell lung cancer is independent of cyclooxygenase expression. Apoptosis 8(6):639–647
Rodriguez-Hernandez A, Brea-Calvo G, Fernandez-Ayala DJ, Cordero M, Navas P, Sanchez-Alcazar JA (2006) Nuclear caspase-3 and caspase-7 activation, and Poly(ADP-ribose) polymerase cleavage are early events in camptothecin-induced apoptosis. Apoptosis 11(1):131–139
Sanchez-Alcazar JA, Khodjakov A, Schneider E (2001) Anticancer drugs induce increased mitochondrial cytochrome c expression that precedes cell death. Cancer Res 61(3):1038–1044
Oakley BR, Akkari YN (1999) Gamma-tubulin at ten: progress and prospects. Cell Struct Funct 24(5):365–372
Andersen SSL (2000) Spindle assembly and the art of regulating microtubule dynamics by MAPs and Stathmin/Op18. Trends in Cell Biology 10(7):261–267
Tokuraku K, Katsuki M, Nakagawa H, Kotani S (1999) A new model for microtubule-associated protein (MAP)-induced microtubule assembly: the Pro-rich region of MAP4 promotes nucleation of microtubule assembly in vitro. Eur J Biochem 259(1):158–166
Tombal B, Denmeade SR, Gillis JM, Isaacs JT (2002) A supramicromolar elevation of intracellular free calcium ([Ca(2+)](i)) is consistently required to induce the execution phase of apoptosis. Cell Death Differ 9(5):561–573
Mattson MP, Chan SL (2003) Calcium orchestrates apoptosis. Nat Cell Biol 5(12):1041–1043
Bonanno E, Ruzittu M, Carla EC, Montinari MR, Pagliara P, Dini L (2000) Cell shape and organelle modification in apoptotic U937 cells. Eur J Histochem 44(3):237–246
Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF (2001) Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol 3(4):339–345
Croft DR, Coleman ML, Li S, Robertson D, Sullivan T, Stewart CL et al (2005) Actin-myosin-based contraction is responsible for apoptotic nuclear disintegration. J Cell Biol 168(2):245–255
Rao L, Perez D, White E (1996) Lamin proteolysis facilitates nuclear events during apoptosis. J Cell Biol 135(6, Part 1):1441–1455
Caulin C, Salvesen GS, Oshima RG (1997) Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J Cell Biol 138(6):1379–1394
Byun Y, Chen F, Chang R, Trivedi M, Green KJ, Cryns VL (2001) Caspase cleavage of vimentin disrupts intermediate filaments and promotes apoptosis. Cell Death And Differ 8(5):443–450
Bonfoco E, Leist M, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P (1996) Cytoskeletal breakdown and apoptosis elicited by NO donors in cerebellar granule cells require NMDA receptor activation. J Neurochem 67(6):2484–2493
Mills JC, Lee VM, Pittman RN (1998) Activation of a PP2A-like phosphatase and dephosphorylation of tau protein characterize onset of the execution phase of apoptosis. J Cell Sci 111(Pt 5):625–636
Janmey PA (1998) The cytoskeleton and cell signaling: component localization and mechanical coupling. Physiol Rev 78(3):763–781
Oshima RG (2002) Apoptosis and keratin intermediate filaments. Cell Death Differ 9(5):486–492
Job D, Valiron O, Oakley B (2003) Microtubule nucleation. Curr Opin Cell Biol 15(1):111–117
Adrain C, Duriez PJ, Brumatti G, Delivani P, Martin SJ (2006) The cytotoxic lymphocyte protease, granzyme B, targets the cytoskeleton and perturbs microtubule polymerization dynamics. J Biol Chem 281(12):8118–8125
Shiina N, Tsukita S (1999) Regulation of microtubule organization during interphase and M phase. Cell Struct Funct 24(5):385–391
Schwab BL, Guerini D, Didszun C, Bano D, Ferrando-May E, Fava E et al (2002) Cleavage of plasma membrane calcium pumps by caspases: a link between apoptosis and necrosis. Cell Death Differ 9(8):818–831
Nicotera P, Orrenius S (1998) The role of calcium in apoptosis. Cell Calcium 23(2–3):173–180
Martin S, Reutelingsperger C, McGahon A, Rader J, van Schie R, LaFace D et al (1995) Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med 182(5):1545–1556
Fadok V, Voelker D, Campbell P, Cohen J, Bratton D, Henson P (1992) Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol 148(7):2207–2216
Hampton MB, Vanags DM, Isabella Porn-Ares M, Orrenius S (1996) Involvement of extracellular calcium in phosphatidylserine exposure during apoptosis. FEBS Lett 399(3):277–282
Bratton DL, Fadok VA, Richter DA, Kailey JM, Guthrie LA, Henson PM (1997) Appearance of phosphatidylserine on apoptotic cells requires calcium-mediated nonspecific flip-flop and is enhanced by loss of the aminophospholipid translocase. J Biol Chem 272(42):26159–26165
Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RAB, Henson PM (2000) A receptor for phosphatidylserine-specific clearance of apoptotic cells. 405(6782):85–90
Verhoven B, Schlegel R, Williamson P (1995) Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J Exp Med 182(5):1597–1601
Acknowledgments
We thank Antonio Arroyo and John Pearson for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Figure S1
Fluorescence microscopy of microtubule in control, CPT, and staurosporine treated primary human fibroblasts. Cells were grown on glass coverslips and treated with CPT or staurosporin for 48 hours. Then cells were fixed and immunostained with mouse anti α-tubulin (red). Nuclear morphology was revealed by staining with Hoechst 33342. Arrows, tubulin reorganization in apoptotic cells. Bar: 30 μm
Figure S2
Fluorescence microscopy of microtubule in control, CPT, and TRAIL treated HeLa cells. Cells were grown on glass coverslips and treated with CPT or TRAIL for 48 hours. Cells were then fixed and immunostained with mouse anti α-tubulin (red). Nuclear morphology was revealed by staining with Hoechst 33342. Arrows, tubulin reorganization in apoptotic cells. Bar: 30 μm
Figure S3
Fluorescence microscopy of microtubule in, CPT, and serum withdrawal treated HeLa cells. Cells were grown on glass coverslips and treated with CPT or serum withdrawal for 48 hours. Cells were fixed and immunostained with mouse anti α-tubulin (red). Nuclear morphology was revealed by staining with Hoechst 33342. Arrows, tubulin reorganization in apoptotic cells. Bar: 30 mm
Figure S4
Fluorescence microscopy of microtubule in control H460 cells. Cells were grown on glass coverslips for 48 hours. Cells were then fixed and immunostained with mouse anti β-tubulin (green). Nuclear morphology was revealed by staining with Hoechst 33342. Arrows, tubulin reorganization in apoptotic cells. Bar = 15 μm.
Figure S5
Light and fluorescence microscopy of CPT-treated H460 apoptotic cells showing AMN, surrounding the cell beneath the plasma membrane. Cells were grown on glass coverslips for 48 hours. Cells were then fixed and immunostained with mouse anti α-tubulin. Nuclear morphology was revealed by staining with Hoechst 33342 Arrow: apoptotic cell.
Figure S6
Light and fluorescence microscopy CPT-treated H460 apoptotic cell showing the AMN extending from body of the cell into slender spikes. Cells were grown on glass coverslips for 48 hours. Cells were then fixed and immunostained with mouse anti β-tubulin (red). Nuclear morphology was revealed by staining with Hoechst 33342.
Figure S7
Fluorescence microscopy of CPT-treated LLCPK-1α cells. Cells were grown on glass coverslips and treated with CPT for 12 hours. Cells were then fixed. Nuclear morphology was revealed by staining with Hoechst 33342. White arrow: apoptotic body with the AMN enclosing a small nuclear fragment. Yellow arrow: large cellular fragment with an AMN.
Figure S8
Fluorescence microscopy of CPT+zVAD treated H460 apoptotic cell showing the AMN in the presence of cytochrome c release (arrow). Cells were grown on glass coverslips for 48 hours. Cells were then fixed and immunostained with rabbit anti α-tubulin (red) and mouse anti cytochrome c. Nuclear morphology was revealed by staining with Hoechst 33342 Nuclei are condensed but not fragmented due to caspase inactivation. Panel A, Bar: 30 mm. Panel B, Bar: 15 μm.
Figure S9
Light and fluorescence microscopy of CPT treated H460 apoptotic cell showing AMN formation and some spots of polymerized actin at the end of the spikes. Cells were grown on glass coverslips for 48 hours. Cells were then fixed and immunostained with mouse anti α-tubulin (green) and TRITC-phalloidin to visualize actin filaments (red). Nuclear morphology was revealed by staining with Hoechst 33342.
Figure S10
Fluorescence microscopy of control and CPT-treated LLCPK-1α cells. Cells were grown on glass coverslips and treated with CPT for 12 hours. Cells were then fixed and stained with and TRITC-phalloidin to visualize actin filaments (red) (red). Nuclear morphology was revealed by staining with Hoechst 33342. Arrow: actin spots in an apoptotic cell with AMN.
Figure S11
LDH release in the medium of Control, CPT, CPT + CYTO, CPT + COL Control + CYTO (X), and Control + COL treated HeLa cells. Cells were treated as described in Material and methods. *, p<0,01, between CPT + COL and CPT treated cells (n=3).
Rights and permissions
About this article
Cite this article
Sánchez-Alcázar, J.A., Rodríguez-Hernández, Á., Cordero, M.D. et al. The apoptotic microtubule network preserves plasma membrane integrity during the execution phase of apoptosis. Apoptosis 12, 1195–1208 (2007). https://doi.org/10.1007/s10495-006-0044-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-006-0044-6