Abstract
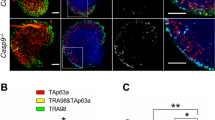
Previously, we analyzed mice lacking either caspase-2 or caspase-3 and documented a role for caspase-2 in developmental and chemotherapy-induced apoptosis of oocytes. Those data also revealed dispensability of caspase-3, although we found this caspase critical for ovarian granulosa cell death. Because of the mutual interdependence of germ cells and granulosa cells, herein we generated caspase-2 and -3 double-mutant (DKO) mice to evaluate how these two caspases functionally relate to each other in orchestrating oocyte apoptosis. No difference was observed in the rate of spontaneous oocyte apoptosis between DKO and wildtype (WT) females. In contrast, the oocytes from DKO females were more susceptible to apoptosis induced by DNA damaging agents, compared with oocytes from WT females. This increased sensitivity to death of DKO oocytes appears to be a specific response to DNA damage, and it was associated with a compensatory upregulation of caspase-12. Interestingly, DKO oocytes were more resistant to apoptosis induced by methotrexate (MTX) than WT oocytes. These results revealed that in female germ cells, insults that directly interfere with their metabolic status (e.g. MTX) require caspase-2 and caspase-3 as obligatory executioners of the ensuing cell death cascade. However, when DNA damage is involved, and in the absence of caspase-2 and -3, caspase-12 becomes upregulated and mediates apoptosis in oocytes.






Similar content being viewed by others
Abbreviations
- BSA:
-
bovine serum albumin
- DKO:
-
double knockout
- DXR:
-
doxorubicin
- eCG:
-
equine chorionic gonadotropin
- ER:
-
endoplasmic reticulum
- hCG:
-
human chorionic gonadotropin
- HTF:
-
human tubal fluid
- LPS:
-
bacterial lipopolysaccharide
- MTX:
-
methotrexate
- PCD:
-
programmed cell death
- WT:
-
wildtype
- z-VAD:
-
pancaspase inhibitor
References
Danial NN, Korsmeyer SJ (2004) Cell death: critical control points. Cell 116:205–219
Fadeel B, Orrenius S (2005) Apoptosis: a basic biological phenomenon with wide-ranging implications in human disease. J Intern Med 258:479–517
Krantic S, Mechawar N, Reix S, Quirion R (2005) Molecular basis of programmed cell death involved in neurodegeneration. Trends Neurosci 28:670–676
Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, Joseph MK, Kitada S, Korsmeyer SJ, Kunzer AR, Letai A, Li C, Mitten MJ, Nettesheim DG, Ng S, Nimmer PM, O’Connor JM, Oleksijew A, Petros AM, Reed JC, Shen W, Tahir SK, Thompson CB, Tomaselli KJ, Wang B, Wendt MD, Zhang H, Fesik SW, Rosenberg SH (2005) An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435:677–681
Reed JC (2006) Proapoptotic multidomain Bcl-2/Bax-family proteins: mechanisms, physiological roles, and therapeutic opportunities. Cell Death Differ [Epub ahead of print]
Takeuchi O, Fisher J, Suh H, Harada H, Malynn BA, Korsmeyer SJ (2005) Essential role of BAX, BAK in B cell homeostasis and prevention of autoimmune disease. Proc Natl Acad Sci USA 102:11272–11277
Lamkanfi, M., Declercq W, Depuydt B, Kalai M, Saelens X, Vandenabeele P (2003) The caspase family. In Los M, Walczak H (eds.) Caspases—Their role in cell death and cell survival. Landes Bioscience and Kluwer Academic, New York, pp. 1–40
Lettre G, Hengartner MO (2006) Developmental apoptosis in C. elegans: a complex CEDnario. Nat Rev Mol Cell Biol 7:97–108
Tilly JL (2001) Commuting the death sentence: how oocytes strive to survive. Nat Rev Mol Cell Biol 2:838–848
De Felici M, Di Carlo A, Pesce M, Iona S, Farrace MG, Piacentini M (1999) Bcl-2 and Bax regulation of apoptosis in germ cells during prenatal oogenesis in the mouse embryo. Cell Death Differ 6:908–915
Jurisicova A, Latham K, Casper RF, Varmuza SL (1998) Expression and regulation of genes associated with cell death during murine preimplantation embryo development. Mol Reprod Dev 51:243–253
Kugu K, Ratts VS, Piquette GN, Tilly KI, Tao X-J, Martimbeau S, Aberdeen GW, Krajewski S, Reed JC, Pepe GJ, Albrecht ED, Tilly JL (1998) Analysis of apoptosis and expression of bcl-2 gene family members in the human and baboon ovary. Cell Death Differ 5:67–76
Tilly JL, Tilly KI, Kenton ML, Johnson AL (1995) Expression of members of the bcl-2 gene family in the immature rat ovary: equine chorionic gonadotropin-mediated inhibition of granulosa cell apoptosis is associated with decreased bax and constitutive bcl-2 and bcl-xlong messenger RNA levels. Endocrinology 136:232–241
Perez GI, Robles R, Knudson CM, Flaws JA, Korsmeyer SJ, Tilly JL (1999) Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nat Genet 21:200–203
Perez GI, Trbovich AM, Gosden RG, Tilly JL (2000) Mitochondria and the death of oocytes. Nature 403:500–501
Yuan J (2006) Divergence from a dedicated cellular suicide mechanism: exploring the evolution of cell death. Mol Cell 23:1–12
Bergeron L, Perez GI, Macdonald G, Shi L, Sun Y, Jurisicova A, Varmuza S, Latham KE, Flaws JA, Salter JC, Hara H, Moskowitz MA, Li E, Greenberg A, Tilly JL, Yuan J (1998) Defects in regulation of apoptosis in caspase-2-deficient mice. Genes Dev 12:1304–1314
Matikainen T, Perez GI, Zheng TS, Kluzak TR, Rueda BR, Flavell RA, Tilly JL (2001) Caspase-3 gene knockout defines cell lineage specificity for programmed cell death signaling in the ovary. Endocrinology 142:2468–2480
Kuida K, Zheng TS, Na S, Kuan C, Yang D, Karasuyama H, Rakic P, Flavell RA (1996) Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature 384:368–372
Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J (2000) Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403:98–103
Jurisicova A, Lee HJ, D’Estaing SG, Tilly JL, Perez GI (2006) Molecular requirements for doxorubicin-mediated death in murine oocytes. Cell Death Differ Jan 27 [Epub ahead of print]
Morita Y, Perez GI, Paris F, Miranda SR, Ehleiter D, Haimovitz-Friedman A, Fuks Z, Xie Z, Reed JC, Schuchman EH, Kolesnick RN, Tilly JL (2000) Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat Med 6:1109–1114
Perez GI, Tao XJ, Tilly JL (1999) Fragmentation and death (a.k.a. apoptosis) of ovulated oocytes. Mol Hum Reprod 5:414–420
Jurisicova A, Antenos M, Varmuza S, Tilly JL, Casper RF (2003) Expression of apoptosis-related genes during human preimplantation embryo development: potential roles for the Harakiri gene product and Caspase-3 in blastomere fragmentation. Mol Hum Reprod 9:133–141
Fujita E, Kouroku Y, Jimbo A, Isoai A, Maruyama K, Momoi T (2002) Caspase-12 processing and fragment translocation into nuclei of tunicamycin-treated cells. Cell Death Differ 9:1108–1114
Kuida K, Haydar TF, Kuan CY, Gu Y, Taya C, Karasuyama H, Su MS, Rakic P, Flavell RA (1998) Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell 94:325–337
Besnault-Mascard L, Leprince C, Auffredou MT, Meunier B, Bourgeade MF, Camonis J, Lorenzo HK, Vazquez A (2005) Caspase-8 sumoylation is associated with nuclear localization. Oncogene 24:3268–3273
Feng Y, Hu J, Xie D, Qin J, Zhong Y, Li X, Xiao W, Wu J, Tao D, Zhang M, Zhu Y, Song Y, Reed E, Li QQ, Gong J (2005) Subcellular localization of caspase-3 activation correlates with changes in apoptotic morphology in MOLT-4 leukemia cells exposed to X-ray irradiation. Int J Oncol 27:699–704
Lakhani SA, Masud A, Kuida K, Porter GA Jr, Booth CJ, Mehal WZ, Inayat I, Flavell RA (2006) Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science 311:785–786
Ruiz-Vela A, Opferman JT, Cheng EH, Korsmeyer SJ (2005) Proapoptotic BAX and BAK control multiple initiator caspases. EMBO Rep 6:379–385
Xu C, Bailly-Maitre B, Reed JC (2005) Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest 115:2656–2664
Zong WX, Li C, Hatzivassiliou G, Lindsten T, Yu QC, Yuan J, Thompson CB (2003) Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J Cell Biol 162:59–69
Lamkanfi M, Kalai M, Vandenabeele P (2004) Caspase-12: an overview. Cell Death Differ 11:365–368
Cheung HH, Lynn-Kelly N, Liston P, Korneluk RG (2006) Involvement of caspase-2 and caspase-9 in endoplasmic reticulum stress-induced apoptosis: A role for the IAPs. Exp Cell Res 312:2347–2357
Eichenlaub-Ritter U (1996) Parental age-related aneuploidy in human germ cells and offspring: a story of past and present. Environ Mol Mutagen 28:211–236
Kirkwood TBL (1998) Ovarian ageing and the general biology of senescence. Maturitas 30:105–111
Lim AST, Tsakok MFH (1997) Age-related decline in fertility: a link to degenerative oocytes? Fertility and Sterility 68:265–271
Wu J, Zhang L, Wang X (2000) Maturation and apoptosis of human oocytes in vitro are age-related. Fertil Steril 74:1137–1141
Hitomi J, Katayama T, Eguchi Y, Kudo T, Taniguchi M, Koyama Y, Manabe T, Yamagishi S, Bando Y, Imaizumi K, Tsujimoto Y, Tohyama M (2004) Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Aß-induced cell death. J Cell Biol 165:347–356.
Fischer H, Koenig U, Eckhart L, Tschachler E (2002) Human caspase 12 has acquired deleterious mutations. Biochem Biophys Res Commun 293:722–726
Zheng TS, Hunot S, Kuida K, Momoi T, Srinivasan A, Nicholson DW, Lazebnik Y, Flavell RA (2000) Deficiency in caspase-9 or caspase-3 induces compensatory caspase activation. Nat Med 6:1241–1247
Fischer U, Schulze-Osthoff K (2005) Apoptosis-based therapies and drug targets. Cell Death Differ 12:942–961
Chan SL, Yu VC (2004) Proteins of the bcl-2 family in apoptosis signalling: from mechanistic insights to therapeutic opportunities. Clin Exp Pharmacol Physiol 31:119–128
Sah NK, Khan Z, Khan GJ, Bisen PS (2006) Structural, functional and therapeutic biology of survivin. Cancer Lett Apr 16 [Epub ahead of print]
Acknowledgments
Supported by the Department of Physiology Michigan State University (GIP), and Vincent Research Funds, Massachusetts General Hospital (GIP). During these studies the following research fellows were supported by various institutions: Y Takai by the Department of OB/GYN, University of Tokyo, Faculty of Medicine (Tokyo, Japan); T Matikainen by the Finnish Foundation for Pediatric Research and the Finnish Cultural Foundation; A Jurisicova was supported by a New Investigator Salary Award from the Canadian Institutes of Health Research; MR Kim by the Department of OB/GYN, The Catholic University of Korea (Seoul, Korea). We would like to thank Mr. Sam Riley (Photo Lab, MGH) for outstanding assistance with the preparation of figures and image analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Takai and Matikainen contributed equally to this work.
Rights and permissions
About this article
Cite this article
Takai, Y., Matikainen, T., Jurisicova, A. et al. Caspase-12 compensates for lack of caspase-2 and caspase-3 in female germ cells. Apoptosis 12, 791–800 (2007). https://doi.org/10.1007/s10495-006-0022-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-006-0022-z