Abstract
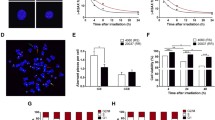
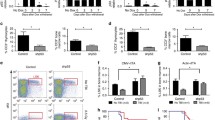
The molecular causes for enhanced radiosensitivity of Nijmegen Breakage Syndrome cells are unclear, especially as repair of DNA damage is hardly impeded in these cells. We clearly demonstrate that radiation hypersensitivity is accompanied by enhanced γ-radiation-induced apoptosis in NBS1 deficient lymphoblastoid cell lines. Differences in the apoptotic behavior of NBS1 −/− and NBS1 +/− cells are not due to an altered p53 stabilization or phosphorylation in NBS1 −/− cells. γ-radiation-induced caspase-8 activity is increased and visualization of CD95 clustering by laser scanning microscopy shows a significant higher activation of the death receptor in NBS1 −/− cells. Further investigation of the molecular mechanisms reveals a role for reactive oxygen species-triggered activation of CD95. These results demonstrate that NBS1 suppresses the CD95 death receptor-dependent apoptotic pathway after γ-irradiation and evidence is given that this is achieved by regulation of the PI3-K/AKT survival pathway.








Similar content being viewed by others
References
Digweed M, Sperling K (2004) Nijmegen breakage syndrome: clinical manifestation of defective response to DNA double-strand breaks. DNA Repair (Amst) 3:1207–1217
Shiloh Y (1997) Ataxia-telangiectasia and the Nijmegen breakage syndrome: related disorders but genes apart. Ann Rev Genet 31:635–662
Tauchi H, Matsuura S, Kobayashi J, Sakamoto S, Komatsu K (2002) Nijmegen breakage syndrome gene, NBS1, and molecular links to factors for genome stability. Oncogene 21: 8967–8980
Buscemi G, Savio C, Zannini L et al (2001) Chk2 activation dependence on Nbs1 after DNA damage. Mol Cell Biol 21: 5214–5222
Yazdi PT, Wang Y, Zhao S, Patel N, Lee EY, Qin J (2002) SMC1 is a downstream effector in the ATM/NBS1 branch of the human S-phase checkpoint. Genes Dev 16:571–582
Jongmans W, Vuillaume M, Chrzanowska K, Smeets D, Sperling K, Hall J (1997) Nijmegen breakage syndrome cells fail to induce the p53-mediated DNA damage response following exposure to ionizing radiation. Mol Cell Biol 17:5016–5022
Huang J, Dynan WS (2002) Reconstitution of the mammalian DNA double-strand break end-joining reaction reveals a requirement for an Mre11/Rad50/NBS1-containing fraction. Nucleic Acids Res 30:667–674
Kraakman-van der Zwet M, Overkamp WJ, Friedl AA et al (1999) Immortalization and characterization of Nijmegen Breakage syndrome fibroblasts. Mutat Res 434:17–27
Riballo E, Kuhne M, Rief N et al (2004) A pathway of double-strand break rejoining dependent upon ATM, Artemis, and proteins locating to gamma-H2AX foci. Mol Cell 16:715–724
Howlett NG, Scuric Z, D’Andrea AD, Schiestl RH (2006) Impaired DNA double strand break repair in cells from Nijmegen breakage syndrome patients. DNA Repair (Amst) 5:251–257
Tauchi H, Kobayashi J, Morishima K et al (2002) Nbs1 is essential for DNA repair by homologous recombination in higher vertebrate cells. Nature 420:93–98
Hickson ID, Davies SL, Davies SM, Robson CN (1990) DNA repair in radiation sensitive mutants of mammalian cells: possible involvement of DNA topoisomerases. Int J Radiat Biol 58:561–568
Tauchi H (2000) Positional cloning and functional analysis of the gene responsible for Nijmegen breakage syndrome, NBS1. J Radiat Res (Tokyo) 41:9–17
Bernstein C, Bernstein H, Payne CM, Garewal H (2002) DNA repair/pro-apoptotic dual-role proteins in five major DNA repair pathways: fail-safe protection against carcinogenesis. Mutat Res 511:145–178
Frappart PO, Tong WM, Demuth I et al (2005) An essential function for NBS1 in the prevention of ataxia and cerebellar defects. Nat Med 11:538–544
Rogoff HA, Pickering MT, Frame FM et al (2004) Apoptosis associated with deregulated E2F activity is dependent on E2F1 and Atm/Nbs1/Chk2. Mol Cell Biol 24:2968–2977
Zhang Y, Lim CU, Williams ES et al (2005) NBS1 knockdown by small interfering RNA increases ionizing radiation mutagenesis and telomere association in human cells. Cancer Res 65:5544–5553
O’Connor L, Harris AW, Strasser A (2000) CD95 (Fas/APO-1) and p53 signal apoptosis independently in diverse cell types. Cancer Res 60:1217–1220
Sheard MA, Uldrijan S, Vojtesek B (2003) Role of p53 in regulating constitutive and X-radiation-inducible CD95 expression and function in carcinoma cells. Cancer Res 63:7176–7184
Owen-Schaub LB, Zhang W, Cusack JC et al (1995) Wild-type human p53 and a temperature-sensitive mutant induce Fas/APO-1 expression. Mol Cell Biol 15:3032–3040
Houston A, O’Connell J (2004) The Fas signalling pathway and its role in the pathogenesis of cancer. Curr Opin Pharmacol 4:321–326
Huang HL, Fang LW, Lu SP, Chou CK, Luh TY, Lai MZ (2003) DNA-damaging reagents induce apoptosis through reactive oxygen species-dependent Fas aggregation. Oncogene 22:8168–8177
Varon R, Vissinga C, Platzer M et al (1998) Nibrin, a novel DNA double-strand break repair protein, is mutated in Nijmegen breakage syndrome. Cell 93:467–476
Demuth I, Frappart PO, Hildebrand G et al (2004) An inducible null mutant murine model of Nijmegen breakage syndrome proves the essential function of NBS1 in chromosomal stability and cell viability. Hum Mol Genet 13:2385–2397
Ueki K, Algenstaedt P, Mauvais-Jarvis F, Kahn CR (2000) Positive and negative regulation of phosphoinositide 3-kinase-dependent signaling pathways by three different gene products of the p85alpha regulatory subunit. Mol Cell Biol 20:8035–8046
Cerosaletti KM, Desai-Mehta A, Yeo TC, Kraakman-Van Der Zwet M, Zdzienicka MZ, Concannon P (2000) Retroviral expression of the NBS1 gene in cultured Nijmegen breakage syndrome cells restores normal radiation sensitivity and nuclear focus formation. Mutagenesis 15:281–286
Lundberg AS, Weinberg RA (1999) Control of the cell cycle and apoptosis. Eur J Cancer 35:1886–1894
Al Rashid ST, Dellaire G, Cuddihy A et al (2005) Evidence for the direct binding of phosphorylated p53 to sites of DNA breaks in vivo. Cancer Res 65:10810–10821
Bader AG, Kang S, Zhao L, Vogt PK (2005) Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer 5:921–929
Osaki M, Oshimura M, Ito H (2004) PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis 9:667–676
Chen YC, Su YN, Chou PC et al (2005) Overexpression of NBS1 contributes to transformation through the activation of phosphatidylinositol 3-kinase/Akt. J Biol Chem 280:32505–32511
Bilderback TR, Gazula VR, Dobrowsky RT (2001) Phosphoinositide 3-kinase regulates crosstalk between Trk A tyrosine kinase and p75(NTR)-dependent sphingolipid signaling pathways. J Neurochem 76:1540–1551
Gulbins E, Grassme H (2002) Ceramide and cell death receptor clustering. Biochim Biophys Acta 1585:139–145
Grassme H, Cremesti A, Kolesnick R, Gulbins E (2003) Ceramide-mediated clustering is required for CD95-DISC formation. Oncogene 22:5457–5470
Kolesnick R, Fuks Z (2003) Radiation and ceramide-induced apoptosis. Oncogene 22:5897–5906
Albanese J, Dainiak N (2000) Regulation of TNFRSF6 (Fas) expression in ataxia telangiectasia cells by ionizing radiation. Radiat Res 154:616–624
Bebb DG, Warrington PJ, de Jong G et al (2001) Radiation induced apoptosis in ataxia telangiectasia homozygote, heterozygote and normal cells. Mutat Res 476:13–20
Takao N, Li Y, Yamamoto K (2000) Protective roles for ATM in cellular response to oxidative stress. FEBS Lett 472:133–136
Takao N, Kato H, Mori R et al (1999) Disruption of ATM in p53-null cells causes multiple functional abnormalities in cellular response to ionizing radiation. Oncogene 18:7002–7009
Lee JH, Paull TT (2004) Direct activation of the ATM protein kinase by the Mre11/Rad50/Nbs1 complex. Science 304:93–96
Uziel T, Lerenthal Y, Moyal L, Andegeko Y, Mittelman L, Shiloh Y (2003) Requirement of the MRN complex for ATM activation by DNA damage. Embo J 22:5612–5621
Dumaz N, Meek DW (1999) Serine15 phosphorylation stimulates p53 transactivation but does not directly influence interaction with HDM2. Embo J 18:7002–7010
Lambert PF, Kashanchi F, Radonovich MF, Shiekhattar R, Brady JN (1998) Phosphorylation of p53 serine 15 increases interaction with CBP. J Biol Chem 273:33048–33053
Difilippantonio S, Celeste A, Fernandez-Capetillo O et al (2005) Role of Nbs1 in the activation of the Atm kinase revealed in humanized mouse models. Nat Cell Biol 7:675–685
Fulda S, Debatin KM (2003) Death receptor signaling in cancer therapy. Curr Med Chem Anti-Canc Agents 3:253–262
Belka C, Marini P, Budach W et al (1998) Radiation-induced apoptosis in human lymphocytes and lymphoma cells critically relies on the up-regulation of CD95/Fas/APO-1 ligand. Radiat Res 149:588–595
Sheard MA (2001) Ionizing radiation as a response-enhancing agent for CD95-mediated apoptosis. Int J Cancer 96:213–220
Hamasu T, Inanami O, Asanuma T, Kuwabara M (2005) Enhanced induction of apoptosis by combined treatment of human carcinoma cells with X rays and death receptor agonists. J Radiat Res (Tokyo) 46:103–110
Kuwabara M, Takahashi K, Inanami O (2003) Induction of apoptosis through the activation of SAPK/JNK followed by the expression of death receptor Fas in X-irradiated cells. J Radiat Res (Tokyo) 44:203–209
Damen JE, Liu L, Rosten P et al (1996) The 145-kDa protein induced to associate with Shc by multiple cytokines is an inositol tetraphosphate and phosphatidylinositol 3,4,5-triphosphate 5-phosphatase. Proc Natl Acad Sci USA 93:1689–1693
Lioubin MN, Algate PA, Tsai S, Carlberg K, Aebersold A, Rohrschneider LR (1996) p150Ship, a signal transduction molecule with inositol polyphosphate-5-phosphatase activity. Genes Dev 10:1084–1095
Maehama T, Dixon JE (1998) The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 273:13375–13378
Myers MP, Pass I, Batty IH et al (1998) The lipid phosphatase activity of PTEN is critical for its tumor supressor function. Proc Natl Acad Sci USA 95:13513–13518
Osaki M, Kase S, Adachi K, Takeda A, Hashimoto K, Ito H (2004) Inhibition of the PI3K-Akt signaling pathway enhances the sensitivity of Fas-mediated apoptosis in human gastric carcinoma cell line, MKN-45. J Cancer Res Clin Oncol 130:8–14
Shiloh Y (2003) ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer 3:155–168
Acknowledgments
The authors thank M. Digweed for the supply of the lymphoblastoid cell lines used for this study. We thank J. Favor, GSF—Institute of Developmental Genetics, for critically reading the manuscript and highly acknowledge the technical help from K. Winkler and N. Kunz.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sagan, D., Mörtl, S., Müller, I. et al. Enhanced CD95-mediated apoptosis contributes to radiation hypersensitivity of NBS lymphoblasts. Apoptosis 12, 753–767 (2007). https://doi.org/10.1007/s10495-006-0021-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-006-0021-0