Abstract
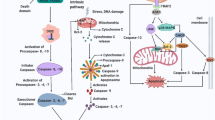
Apoptosis is characterized by chromatin condensation, DNA cleavage, redistribution of phosphatidylserine, and apoptotic body formation via an actin-dependent process. We describe a novel form of the execution phase of apoptosis in human multiple myeloma cells that is morphologically and mechanistically distinct from classical apoptosis, but is caspase-dependent and inhibited by IL-6 and overexpression of Bcl-2. Electron microscopic analysis of these cells demonstrated chromatin condensation without nuclear fragmentation, and ‘partitioning’ of cell constituents into two components: a single, large bleb containing soluble protein and free ribosomes, and a region containing the nucleus, organelles, and RER. In some cases, the bleb separated, becoming a free vesicle exhibiting random kinetic motion. These morphologic features occurred despite inhibition of the actin and tubulin cytoskeletal systems. This novel form of apoptosis, called partitioning apoptosis, was observed in a variety of tumor cell types and in primary cells. The execution phase of apoptosis can occur in a manner that is morphologically and mechanistically distinct from classical apoptosis.
Similar content being viewed by others
References
Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: A link between cancer genetics and chemotherapy. Cell 2002; 108: 153–164.
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100: 57–70.
Arends MJ, Wyllie AH. Apoptosis: Mechanisms and roles in pathology. Int Rev Exp Pathol 1991; 32: 223–254.
Wyllie AH, Kerr JF, Currie AR. Cell death: The significance of apoptosis. Int Rev Cytol 1980; 68: 251–306.
Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature 2000; 405: 85–90.
Liu X, Zou H, Slaughter C, Wang X. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell 1997; 89: 175–184.
Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 1998; 391: 43–50.
Rao L, Perez D, White E. Lamin proteolysis facilitates nuclear events during apoptosis. J Cell Biol 1996; 135: 1441–1455.
Lee N, MacDonald H, Reinhard C, et al. Activation of hPAK65 by caspase cleavage induces some of the morphological and biochemical changes of apoptosis. Proc Natl Acad Sci USA 1997; 94: 13642–13647.
Rudel T, Bokoch GM. Membrane and morphological changes in apoptotic cells regulated by caspase-mediated activation of PAK2. Science 1997; 276: 1571–1574.
Kothakota S, Azuma T, Reinhard C, et al. Caspase-3-generated fragment of gelsolin: Effector of morphological change in apoptosis. Science 1997; 278: 294–298.
Zheng TS, Schlosser SF, Dao T, et al. Caspase-3 controls both cytoplasmic and nuclear events associated with Fas-mediated apoptosis in vivo. Proc Natl Acad Sci USA 1998; 95: 13618–13623.
Janicke RU, Sprengart ML, Wati MR, Porter AG. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem 1998; 273: 9357– 9360.
Martin SJ, O’Brien GA, Nishioka WK, et al. Proteolysis of fodrin (non-erythroid spectrin) during apoptosis. J Biol Chem 1995; 270: 6425–6428.
Cotter TG, Lennon SV, Glynn JM, Green DR. Microfilament-disrupting agents prevent the formation of apoptotic bodies in tumor cells undergoing apoptosis [published erratum appears in Cancer Res 1992;52(12): 3512]. Cancer Res 1992; 52: 997–1005
Laster SM, Mackenzie JM, Jr. Bleb formation and F-actin distribution during mitosis and tumor necrosis factor-induced apoptosis. Microsc Res Tech 1996; 34: 272–280.
Mills JC, Stone NL, Erhardt J, Pittman RN. Apoptotic membrane blebbing is regulated by myosin light chain phosphorylation. J Cell Biol 1998; 140: 627–636.
Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol 2001; 3: 339–345.
Sebbagh M, Renvoize C, Hamelin J, Riche N, Bertoglio J, Breard J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol 2001; 3: 346–352.
Zhang B, Gojo I, Fenton RG. Myeloid cell factor-1 is a critical survival factor for multiple myeloma. Blood 2002; 99: 1885–1893.
Jelinek DF. Mechanisms of myeloma cell growth control. Hematol Oncol Clin North Am 1999; 13: 1145–1157.
Klein B, Zhang XG, Jourdan M, et al. Paracrine rather than autocrine regulation of myeloma-cell growth and differentiation by interleukin-6. Blood 1989; 73: 517–526.
Gojo I, Zhang B, Fenton RG. The Cyclin-dependent Kinase Inhibitor Flavopiridol Induces Apoptosis in Multiple Myeloma Cells through Transcriptional Repression and Down-Regulation of Mcl-1. Clin Cancer Res 2002; 8: 3527–3538.
Vanags DM, Porn-Ares MI, Coppola S, Burgess DH, Orrenius S. Protease involvement in fodrin cleavage and phosphatidylserine exposure in apoptosis. J Biol Chem 1996; 271: 31075–31085.
Mills JC, Stone NL, Pittman RN. Extranuclear apoptosis. The role of the cytoplasm in the execution phase. J Cell Biol 1999; 146: 703–708.
Shimizu T, Cao CX, Shao RG, Pommier Y. Lamin B phosphorylation by protein kinase calpha and proteolysis during apoptosis in human leukemia HL60 cells. J Biol Chem 1998; 273: 8669–8674.
Henson PM, Bratton DL, Fadok VA. The phosphatidylserine receptor: A crucial molecular switch? Nat Rev Mol Cell Biol 2001; 2: 627–633.
Watters D, Waterhouse N. Proteolytic targets in cell death. Results Probl Cell Differ 1998; 24: 25–44.
Robertson JD, Orrenius S, Zhivotovsky B. Review: Nuclear events in apoptosis. J Struct Biol 2000; 129: 346–358.
Martelli AM, Zweyer M, Ochs RL, et al. Nuclear apoptotic changes: An overview. J Cell Biochem 2001; 82: 634– 646.
Stolzenberg I, Wulf S, Mannherz HG, Paddenberg R. Different sublines of Jurkat cells respond with varying susceptibility of internucleosomal DNA degradation to different mediators of apoptosis. Cell Tissue Res 2000; 301: 273–282.
Faleiro L, Lazebnik Y. Caspases disrupt the nuclear-cytoplasmic barrier. J Cell Biol 2000; 151: 951–959.
Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol 1995; 146: 3–15.
Hirsch T, Marchetti P, Susin SA, et al. The apoptosis-necrosis paradox. Apoptogenic proteases activated after mitochondrial permeability transition determine the mode of cell death. Oncogene 1997; 15: 1573–1581.
Leist M, Jaattela M. Four deaths and a funeral: From caspases to alternative mechanisms. Nat Rev Mol Cell Biol 2001; 2: 589–598.
Green DR, Reed JC. Mitochondria and apoptosis. Science 1998; 281:1309–1312.
Leist M, Nicotera P. The shape of cell death. Biochem Biophys Res Commun 1997; 236: 1–9.
McCarthy NJ, Whyte MK, Gilbert CS, Evan GI. Inhibition of Ced-3/ICE-related proteases does not prevent cell death induced by oncogenes, DNA damage, or the Bcl-2 homologue Bak. J Cell Biol 1997; 136: 215–227.
Hoffmann PR, deCathelineau AM, Ogden CA, et al. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J Cell Biol 2001; 155: 649–659.
Somersan S, Bhardwaj N. Tethering and tickling: A new role for the phosphatidylserine receptor. J Cell Biol 2001; 155: 501–504.
Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: Exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med 2000; 191: 423–434.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, B., Arany, Z., Mann, D. et al. Partitioning apoptosis: A novel form of the execution phase of apoptosis. Apoptosis 10, 219–231 (2005). https://doi.org/10.1007/s10495-005-6077-4
Issue Date:
DOI: https://doi.org/10.1007/s10495-005-6077-4