Abstract
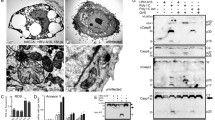
Viral double-stranded RNA (dsRNA) is a ubiquitous intracellular “alert signal” used by cells to detect viral infection and to mount anti-viral responses. DsRNA triggers a rapid (complete within 2–4 h) apoptosis in the highly-susceptible HeLa cell line. Here, we demonstrate that the apical event in this apoptotic cascade is the activation of procaspase 8. Downstream of caspase 8, the apoptotic signaling cascade bifurcates into a mitochondria-independent caspase 8/caspase 3 arm and a mitochondria-dependent, caspase 8/Bid/Bax/Bak/cytochrome c arm. Both arms impinge upon, and activate, procaspase 9 via two different cleavage sites within the procaspase 9 molecule (D330 and D315, respectively). This is the first in vivo demonstration that the “effector” caspase 3 plays an “initiator” role in the regulation of caspase 9. The dsRNA-induced apoptosis is potentiated by the inhibition of protein synthesis, whose role is to accelerate the execution of all apoptosis steps downstream of, and including, the activation of caspase 8. Thus, efficient apoptosis in response to viral dsRNA results from the co-operation of the two major apical caspases (8 and 9) and the dsRNA-activated protein kinase R (PKR)/ribonuclease L (RNase L) system that is essential for the inhibition of protein synthesis in response to viral infection.
Similar content being viewed by others
References
Jacobs BL, Langland JO. When two strands are better than one: The mediators and modulators of the cellular responses to double-stranded RNA. Virology 1996; 219: 339–349.
Chu WM, Ostertag D, Li ZW, et al. JNK2 and IKKbeta are required for activating the innate response to viral infection. Immunity 1999; 11: 721–731.
Iordanov MS, Paranjape JM, Zhou A, et al. Activation of p38 mitogen-activated protein kinase and c-Jun NH(2)-terminal kinase by double-stranded RNA and encephalomyocarditis virus: Involvement of RNase L, protein kinase R, and alternative pathways. Mol Cell Biol 2000; 20: 617– 627.
Iordanov MS, Wong J, Bell JC, Magun BE. Activation of NF-κB by double-stranded RNA (dsRNA) in the absence of protein kinase R and RNase L demonstrates the existence of two separate dsRNA-triggered antiviral programs. Mol Cell Biol 2001; 21: 61–72.
Balachandran S, Kim CN, Yeh WC, Mak TW, Bhalla K, Barber GN. Activation of the dsRNA-dependent protein kinase, PKR, induces apoptosis through FADD-mediated death signaling. Embo J 1998; 17: 6888–6902.
Iordanov MS, Ryabinina OP, Wong J, et al. Molecular determinants of apoptosis induced by the cytotoxic ribonuclease onconase: Evidence for cytotoxic mechanisms different from inhibition of protein synthesis. Cancer Res 2000; 60: 1983–1994.
Liu D, Cardozo AK, Darville MI, Eizirik DL. Double-stranded RNA cooperates with interferon-gamma and IL-1 beta to induce both chemokine expression and nuclear factor-κ B-dependent apoptosis in pancreatic beta-cells: Potential mechanisms for viral-induced insulitis and beta-cell death in type 1 diabetes mellitus. Endocrinology 2002; 143: 1225–1234.
Barber GN. Host defense, viruses and apoptosis. Cell Death Differ 2001; 8: 113–126.
Donze O, Dostie J, Sonenberg N. Regulatable expression of the interferon-induced double-stranded RNA dependent protein kinase PKR induces apoptosis and fas receptor expression. Virology 1999; 256: 322–329.
Kibler KV, Shors T, Perkins KB, et al. Double-stranded RNA is a trigger for apoptosis in vaccinia virus-infected cells. J Virol 1997; 71: 1992–2003.
Teodoro JG, Branton PE. Regulation of apoptosis by viral gene products. J Virol 1997; 71: 1739–1746.
Cuconati A, White E. Viral homologs of BCL-2: Role of apoptosis in the regulation of virus infection. Genes Dev 2002; 16: 2465–2478.
Schwarz EM, Badorff C, Hiura TS, et al. NF-κB-mediated inhibition of apoptosis is required for encephalomyocarditis virus virulence: A mechanism of resistance in p50 knockout mice. J Virol 1998; 72: 5654–5660.
Kerr JF, Wyllie AH, Currie AR. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 1972; 26: 239–257.
Hengartner MO. The biochemistry of apoptosis. Nature 2000; 407: 770–776.
Rich T, Allen RL, Wyllie AH. Defying death after DNA damage. Nature 2000; 407: 777–783.
Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature 2000; 407: 784–788.
Krammer PH. CD95’s deadly mission in the immune system. Nature 2000; 407: 789–795.
Meier P, Finch A, Evan G. Apoptosis in development. Nature 2000; 407: 796–801.
Yuan J, Yankner BA. Apoptosis in the nervous system. Nature 2000; 407: 802–809.
Nicholson DW. From bench to clinic with apoptosis-based therapeutic agents. Nature 2000; 407: 810–816.
Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell 2001; 104: 487–501.
Chinnaiyan AM, O’Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 1995; 81: 505–512.
Boldin MP, Varfolomeev EE, Pancer Z, Mett IL, Camonis JH, Wallach D. A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J Biol Chem 1995; 270: 7795–7798.
Boldin MP, Goncharov TM, Goltsev YV, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell 1996; 85: 803–815.
Muzio M, Chinnaiyan AM, Kischkel FC, et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling. Cell 1996; 85: 817–827.
Kischkel FC, Hellbardt S, Behrmann I, et al., Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. Embo J 1995; 14: 5579–5588.
Muzio M, Stockwell BR, Stennicke HR, Salvesen GS, Dixit VM. An induced proximity model for caspase-8 activation. J Biol Chem 1998; 273: 2926–2930.
Ferri KF, Kroemer G. Mitochondria-the suicide organelles. Bioessays 2001; 23: 111–115.
Ferri KF, Kroemer G. Organelle-specific initiation of cell death pathways. Nat Cell Biol 2001; 3: E255–263.
Wang X. The expanding role of mitochondria in apoptosis. Genes Dev 2001; 15: 2922–2933.
Zou H, Li Y, Liu X, Wang X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem 1999; 274: 11549–11556.
Acehan, D, Jiang X, Morgan DG, Heuser JE, Wang X, Akey CW. Three-dimensional structure of the apoptosome: Implications for assembly, procaspase-9 binding, and activation. Mol Cell 2002; 9: 423–432.
Srinivasula SM, Fernandes-Alnemri T, Zangrilli J, et al. The Ced-3/interleukin 1beta converting enzyme-like homolog Mch6 and the lamin-cleaving enzyme Mch2alpha are substrates for the apoptotic mediator CPP32. J Biol Chem 1996; 271: 27099–27106.
Duan H, Orth K, Chinnaiyan AM, et al. ICE-LAP6, a novel member of the ICE/Ced-3 gene family, is activated by the cytotoxic T cell protease granzyme B. J Biol Chem 1996; 271: 16720–16724.
Srinivasula SM, Ahmad M, Fernandes-Alnemri T, Alnemri ES. Autoactivation of procaspase-9 by Apaf-1-mediated oligomerization. Mol Cell 1998; 1: 949–957.
Cohen GM. Caspases: The executioners of apoptosis. Biochem J 1997; 326(Pt 1): 1–16.
Shi Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell 2002; 9: 459–470.
Luo X, Budihardjo I, Zou H, Slaughter C, and Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 1998; 94: 481–490.
Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 1998; 94: 491–501.
Wei MC, Zong WX, Cheng EH, et al. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science 2001; 292: 727–730.
Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ 2000; 7: 1166–1173.
Krajewski S, Krajewska M, Reed JC. Immunohistochemical analysis of in vivo patterns of Bak expression, a proapoptotic member of the Bcl-2 protein family. Cancer Res 1996; 56: 2849–2855.
Griffiths GJ, Dubrez L, Morgan CP, et al. Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis. J Cell Biol 1999; 144: 903–914.
Nechushtan A, Smith CL, Lamensdorf I, Yoon SH, Youle RJ. Bax and Bak coalesce into novel mitochondria-associated clusters during apoptosis. J Cell Biol 2001; 153: 1265–1276.
Wang GQ, Gastman BR, Wieckowski E, et al. A role for mitochondrial Bak in apoptotic response to anticancer drugs. J Biol Chem 2001; 276: 34307–34317.
Wei MC, Lindsten T, Mootha VK, et al. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev 2000; 14: 2060–2071.
Balachandran S, Roberts PC, Kipperman T, et al. Alpha/beta interferons potentiate virus-induced apoptosis through activation of the FADD/Caspase-8 death signaling pathway. J Virol 2000; 74: 1513–1523.
Bratton SB, Walker G, Srinivasula SM, et al. Recruitment, activation and retention of caspases-9 and -3 by Apaf-1 apoptosome and associated XIAP complexes. Embo J 2001; 20: 998–1009.
Roy S, Bayly CI, Gareau Y, et al. Maintenance of caspase-3 proenzyme dormancy by an intrinsic “safety catch” regulatory tripeptide. Proc Natl Acad Sci USA 2001; 98: 6132–6137.
Thornberry NA, Rano TA, Peterson EP, et al. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem 1997; 272: 17907–17911.
Garcia-Calvo M, Peterson EP, Leiting B, Ruel R, Nicholson DW, Thornberry NA. Inhibition of human caspases by peptide-based and macromolecular inhibitors. J Biol Chem 1998; 273: 32608–32613.
Li F, Srinivasan A, Wang Y, Armstrong RC, Tomaselli KJ, Fritz LC. Cell-specific induction of apoptosis by microinjection of cytochrome c. Bcl-xL has activity independent of cytochrome c release. J Biol Chem 1997; 272: 30299–30305.
Srinivasula SM, Hegde R, Saleh A, et al. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature 2001; 410: 112–116.
Meurs E, Chong K, Galabru J, et al. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell 1990; 62: 379–390.
Levin D, London IM. Regulation of protein synthesis: Activation by double-stranded RNA of a protein kinase that phosphorylates eukaryotic initiation factor 2. Proc Natl Acad Sci USA 1978; 75: 1121–1125.
Clemens MJ. Regulation of eukaryotic protein synthesis by protein kinases that phosphorylate initiation factor eIF-2. Mol Biol Rep 1994; 19: 201–210.
Chebath J, Benech P, Hovanessian A, Galabru J, Revel M. Four different forms of interferon-induced 2′,5′-oligo(A) synthetase identified by immunoblotting in human cells. J Biol Chem 1987; 262: 3852–3857.
Zhou A, Hassel BA, Silverman RH. Expression cloning of 2-5A-dependent RNAase: A uniquely regulated mediator of interferon action. Cell 1993; 72: 753–765.
Silverman RH, Cayley PJ, Knight M, Gilbert CS, Kerr IM. Control of the ppp(a2′p)nA system in HeLa cells. Effects of interferon and virus infection. Eur J Biochem 1982; 124: 131–138.
Wreschner DH, James TC, Silverman RH, Kerr IM. Ribosomal RNA cleavage, nuclease activation and 2-5A(ppp(A2′p)nA) in interferon-treated cells. Nucleic Acids Res 1981; 9: 1571–1581.
Gil J, Alcami J, Esteban M. Induction of apoptosis by double-stranded-RNA-dependent protein kinase (PKR) involves the alpha subunit of eukaryotic translation initiation factor 2 and NF-κB. Mol Cell Biol 1999; 19: 4653–4663.
Lee SB, Rodriguez D, Rodriguez JR, Esteban M. The apoptosis pathway triggered by the interferon-induced protein kinase PKR requires the third basic domain, initiates upstream of Bcl-2, and involves ICE-like proteases. Virology 1997; 231: 81–88.
Srivastava SP, Kumar KU, Kaufman RJ. Phosphorylation of eukaryotic translation initiation factor 2 mediates apoptosis in response to activation of the double-stranded RNA-dependent protein kinase. J Biol Chem 1998; 273: 2416–2423.
Yeung MC, Chang DL, Camantigue RE, Lau AS. Inhibitory role of the host apoptogenic gene PKR in the establishment of persistent infection by encephalomyocarditis virus in U937 cells. Proc Natl Acad Sci USA 1999; 96: 11860–11865.
Castelli J, Wood KA, Youle RJ. The 2-5A system in viral infection and apoptosis. Biomed Pharmacother 1998 52: 386–390.
Castelli JC, Hassel BA, Maran A, et al. The role of 2′-5′ oligoadenylate-activated ribonuclease L in apoptosis. Cell Death Differ 1998; 5: 313–320.
Castelli JC, Hassel BA, Wood KA, et al. A study of the interferon antiviral mechanism: Apoptosis activation by the 2-5A system. J Exp Med 1997; 186: 967–972.
Zhou A, Paranjape J, Brown TL, et al. Interferon action and apoptosis are defective in mice devoid of 2′,5′-oligoadenylate-dependent RNase L. Embo J 1997; 16: 6355–6363.
Zhou A, Paranjape JM, Hassel BA, et al. Impact of RNase L overexpression on viral and cellular growth and death. J Interferon Cytokine Res 1998; 18: 953–961.
Kreuz S, Siegmund D, Scheurich P, Wajant H. NF-κB inducers upregulate cFLIP, a cycloheximide-sensitive inhibitor of death receptor signaling. Mol Cell Biol 2001; 21: 3964–3973.
Iordanov MS, Pribnow D, Magun JL, Dinh TH, Pearson JA, Magun BE. Ultraviolet radiation triggers the ribotoxic stress response in mammalian cells. J Biol Chem 1998; 273: 15794–15803.
Iordanov MS, Magun BE. Loss of cellular K+ mimics ribotoxic stress. Inhibition of protein synthesis and activation of the stress kinases SEK1/MKK4, stress-activated protein kinase/c-Jun NH2-terminal kinase 1, and p38/HOG1 by palytoxin. J Biol Chem 1999; 274: 25801–25806.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by U.S. Public Health Service Grants CA-39360 and ES-08456 to B.E.M. and CA-93718 to M.S.I.
Rights and permissions
About this article
Cite this article
Iordanov, M.S., Ryabinina, O.P., Schneider, P. et al. Two mechanisms of caspase 9 processing in double-stranded RNA- and virus-triggered apoptosis. Apoptosis 10, 153–166 (2005). https://doi.org/10.1007/s10495-005-6070-y
Issue Date:
DOI: https://doi.org/10.1007/s10495-005-6070-y