Abstract
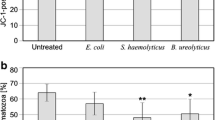
An increased number of sperm undergoing apoptosis has been observed during inflammatory processes in the male genital tract, which might be associated with elevated reactive oxygen species (ROS) levels. However, another factor to stimulate apoptosis could be the direct contact with bacteria or its products, even in the absence of ROS. The aim of this study was to investigate whether bacteria can directly initiate apoptosis in human spermatozoa. Human spermatozoa selected by density gradient centrifugation were incubated with polymorphonuclear granulocytes (PMN) isolated from blood and/or E. faecalis, E. coli or S. aureus. As ROS inductor in PMN, phorbol-12-myristate-13-acetate was used. After incubating the cells for 60 min at 37∘C, ROS were determined by chemiluminescence and phosphatidyl serine (PS) externalization was analyzed by flow cytometry with Annexin V-FITC and propidium iodide (PI). The increase in the percentage of spermatozoa Annexin V-FITC-positive/ PI-negative (early event of late apoptosis) was significant after the incubation with PMN plus PMA, PMN plus E. coli and E. coli alone. The percentage of spermatozoa Annexin V-FITC-positive/ PI-positive (apoptosis/necrosis) increased significantly in sperm incubated with E. coli and S. aureus(20.3% ± 3 and 13.6% ± 3.2 compared to sperm alone, 6% ± 0.5). Sperm incubated with PMN-PMA activated showed only a relative increase in apoptosis/necrosis (8.4% ± 1). Our results show that bacteria directly increase the PS externalisation in ejaculated human sperm. This way of inducing apoptosis does not require external ROS and may result from anyone of the molecular mechanisms that account for changes in motility, vitality and DNA integrity, that are characteristics of spermatozoa in male genital tract infection.
Similar content being viewed by others
References
Sinha Hikim AP, Wang C, Lue Y, et al. Spontaneus germ cell apoptosis in humans: Evidence of ethnic differences in the susceptibility of germ cells to programmed cell death. J Clin Endocrinol Metab 1998; 83: 152–156.
Weng SL, Taylor SL, Morshedi M, et al. Caspase activity and apoptotic markers in ejaculated human sperm. Mol Hum Reprod 2002; 8: 984–991.
Lee J, Richburg JH, Younkin SC, Boekelheide K. The Fas system is a key regulator of germ cell apoptosis in the testis. Endocrinology 1997; 138: 2081–2088.
Lopes S, Jurisicova A, Sun JG, Casper RF. Reactive oxygen species: Potential cause for DNA fragmentation in human spermatozoa. Hum Reprod 1998; 13: 896–900.
Ochsendorf FR. Infection and reactive oxygen species. Andrologia 1997; 30 (Suppl. 1): 81–86.
Crane JK, Majumdar S, Pickhardt III D. Host cell death due to enteropathogenic Escherichia coli has features of apoptosis. Infect Immun 1999; 67: 2575–2584.
Ching JCY, Jones N, Ceponis PJM, Karmali MA, Sherman P. Escherichia coli induce apoptosis and cleavage of poly (ADP-Ribosa) polymerase via in vitro activation of caspases. Infect Immunol 2002; 70: 4669–4677.
Chen Y, Zychlinsky A. Apoptosis induced by bacterial pathogens. Microbial Path 1994; 17: 203–212.
Grasmé H, Jendrossek V, Gulbins E. Molecular mechanisms of bacteria induced apoptosis. Apoptosis 2001; 6: 441–445.
Creagh EM, Martin SJ. Caspases: Cellular demolition experts. Bioch Soc Trans 2001; 29: 696–702.
Erenpreiss J, Hlevicka S, Zalkalns J, Erenpreisa J. Effect of leukocytospermia on sperm DNA integrity: A negative effect in abnormal samen samples. J Androl 2002; 23: 717– 723.
Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril 2003; 79: 829–843.
Koopman G, Reutelingsperger CP, Kuijten GA, et al. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 1994; 84: 1415–1420.
Vernes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods 1995; 184: 39– 41.
World Health Organization. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4th edn., Cambridge, UK 1999; 4–22.
Quinn P, Kerin JF, Warnes GM. Improved pregnancy rate in human in vitro fertilization with the use of a medium based on the composition of human tubular fluid. Fertil Steril 1985; 44: 493–498.
Boyum A. Separation of lymphocytes and erythrocytes by centrifugation. Scand J Clin Invest 1968; 21: 77–85.
Gorga F, Galdiero M, Buommino E, Galdiero E. Porins and lipopolysaccharide induce apoptosis in human spermatozoa. Clin Diagn Lab Immunol 2002; 8: 206–208.
Sánchez R, Villagrán E, Concha M, Cornejo R. Ultrastructural analysis of the attachment sites of Escherichia coli to the human spermatozoon after in vitro migration through estrogenic cervical mucus. Int J Fertil 1989; 34: 363–367.
Jonas D, Walev I, Berger T. Novel path to apoptosis: Small transmembrane pores created by staphylococcal alpha-toxin in T lymphocytes evoke internucleosomal DNA degradation. Infect Immunol 1994; 62: 1304–1312.
Esen M, Schreiner B, Jendrossek V, et al. Mechanisms of Staphylococcus aureus induced apoptosis of human endothelial cells. Apoptosis 2001; 6: 431-439.
Jones NL, Islur A, Haq R, et al. Escherichia coli Shiga toxins induce apoptosis in epithelial cells that is regulated by the Bcl-2 family. Am J Physiol 2000; 278: G811–G819.
Bartoov B, Ozbonfil D, Maayan MC, Ohad E, Nitzan Y. Virulence characteristics of male genital tract Escherichia coli isolated from semen of suspected infertile men. Andrologia 1991; 23: 387–394.
Abul-Milh M, Wu Y, Lau B, Lingwood CA, Barnett-Foster D. Induction of epithelial cell death including apoptosis by enteropathogenic Escherichia coli expressing bundle-forming pili. Infect Immunol 2001; 69: 7356–7364.
Blomgram R, Zheng L, Stendahl O. Uropathogenic Escherichia coli triggers oxygen-dependent apoptosis in human neutrophils through the cooperative effect of type 1 fimbriae and lipopolysaccharide. Infect Immun 2004; 72: 4570–4578.
Wolff H, Panhans A, Stolz W, Meaurer M. Adherence of Escherichia coli to sperm: A mannose mediated phenomenon leading agglutination of sperm and E. coli. Fertil Steril 1993; 60: 154–158.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Villegas, J., Schulz, M., Soto, L. et al. Bacteria induce expression of apoptosis in human spermatozoa. Apoptosis 10, 105–110 (2005). https://doi.org/10.1007/s10495-005-6065-8
Issue Date:
DOI: https://doi.org/10.1007/s10495-005-6065-8