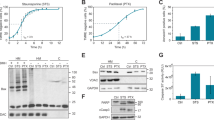
DZ, a benzodiazepine known to affect centrosome separation at prophase, leads to a higher degree of mitotic arrest in HeLa cells than in primary human fibroblasts. In fact, differently from fibroblasts, which undergo a transient block in prophase-to-prometaphase transition, a high proportion of tumor cells attempt to escape from the DZ-imposed mitotic block, fail to undergo complete mitosis and die by mitotic failure. DZ-treated samples showed certain biochemical hallmarks of apoptosis, such as induction of the proapototic Bax protein, mitochondrial alterations assessed by JC-1 staining and TEM analysis, PARP cleavage, and DNA fragmentation. However, in DZ-treated cells, we observed a very low or absent caspase activation as shown by immunofluorescence and immunoblot experiments with antibodies directed to activated caspases and by staining with the pancaspase inhibitor FITC-VAD-FMK. Experiments on mitochondrial depolymerization and apoptosis induction carried out in the presence of specific inhibitors of caspase-2 and caspase-3/7 indicated a caspase-independent apoptotic process induced by DZ. Accordingly, TEM analysis of treated cells revealed ultrastructural features resembling those reported for caspase-independent apoptosis. In conclusion, we hypothesize that HeLa cells override the prophase block imposed by DZ, producing a high rate of aberrant pro-metaphases, which, in turn, activates caspase-independent, apoptosis-like mitotic catastrophe.
Similar content being viewed by others
References
Strasser A, O’Connor L, Dixit VM. Apoptosis signaling. Annu Rev Biochem 2000; 69(2): 17–45.
Leist M, Jaattela M. Four deaths and a funeral: From caspases to alternative mechanisms. Nat Rev Mol Cell Biol 2001; 2: 589–598.
Jaattela M. Multiple cell death pathways as regulators of tumour initiation and progression. Oncogene 2004; 23: 2746–2756.
Hengartner MO. The biochemistry of apoptosis. Nature 2000; 407: 770–776.
Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 1999; 399: 483–487. Erratum in: Nature 407: 767.
Mollinedo F, Gajate C. Microtubules, microtubule-interfering agents and apoptosis. Apoptosis 2003; 8: 413–450.
Cogswell JP, Brown CE, Bisi JE, Neill SD. Dominant-negative polo-like kinase 1 induces mitotic catastrophe independent of cdc25C function. Cell Growth Differ 2000; 11: 615–623.
Sato N, Mizumoto K, Nakamura M, et al. A possible role for centrosome overduplication in radiation-induced cell death. Oncogene 2000; 19: 5281–5290.
Castedo M, Perfettini JL, Roumier T, Andreau K, Medema R, Kroemer G. Cell death by mitotic catastrophe: A molecular definition. Oncogene 2004; 23: 2825–2837.
Swanson PE, Carroll SB, Zhang XF, Mackey MA. Spontaneous premature chromosome condensation, micronucleus formation, and non-apoptotic cell death in heated HeLa S3 cells. Ultrastructural observations. Am J Pathol 1995; 146: 963–971.
Ianzini F, Mackey MA. Spontaneous premature chromosome condensation and mitotic catastrophe following irradiation of HeLa S3 cells. Int J Radiat Biol 1997; 72: 409–421.
Tsvetkov L, Xu X, Li J, Stern DF. Polo-like kinase 1 and Chk2 interact and co-localize to centrosomes and the midbody. J Biol Chem 2003; 278: 8468–8475.
Chehab NH, Malikzay A, Appel M, Halazonetis TD. Chk2/hCds1 functions as a DNA damage checkpoint in G(1) by stabilizing p53. Genes Dev 2000; 14: 278–288.
Peng CY, Graves PR, Thoma RS, Wu Z, Shaw AS. Piwnica-Worms. Mitotic and G2 checkpoint control: Regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science 1997; 277: 1501–1505.
Seo GJ, Kim SE, Lee YM, Lee JW, Lee JR, Hahn MJ, Kim ST. Determination of substrate specificity and putative substrates of Chk2 kinase. Biochem Biophys Res Commun 2003; 304: 339–343.
Broker LE, Huisman C, Ferreira CG, Rodriguez JA, Kruyt FA, Giaccone G. Late activation of apoptotic pathways plays a negligible role in mediating the cytotoxic effects of discodermolide and epothilone B in non-small cell lung cancer cells. Cancer Res 2002; 62: 4081–4088.
Huisman C, Ferreira CG, Broker LE, et al. Paclitaxel triggers cell death primarily via caspase-independent routes in the non-small cell lung cancer cell line NCI-H460. Clin Cancer Res 2002; 8: 596–606.
Nabha SM, Mohammad RM, Dandashi MH, et al. Combretastatin-A4 prodrug induces mitotic catastrophe in chronic lymphocytic leukemia cell line independent of caspase activation and poly(ADP-ribose) polymerase cleavage. Clin Cancer Res 2002; 8: 2735–2741.
Broker LE, Huisman C, Span SW, Rodriguez JA, Kruyt FA, Giaccone G. Cathepsin B mediates caspase-independent cell death induced by microtubule stabilizing agents in non-small cell lung cancer cells. Cancer Res 2004; 64: 27–30.
Kagawa S, Gu J, Honda T, McDonnell TJ, Swisher SG, Roth JA, Fang B. Deficiency of caspase-3 in MCF7 cells blocks Bax-mediated nuclear fragmentation but not cell death. Clin Cancer Res 2001; 7: 1474–1480.
Susin SA, Lorenzo HK, Zamzami N, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 1999; 397: 441–446.
Volbracht C, Leist M, Kolb SA, Nicotera P. Apoptosis in caspase-inhibited neurons. Mol Med 2001; 7: 36–48.
Lockshin RA, Zakeri Z. Caspase-independent cell death? Oncogene 2004; 23: 2766–2773.
Byck R. Drugs and the treatment of psychiatric disorders. In: Goodman LS, Gilman A, eds The Pharmacological Basis of Therapeutics, New York: Edn. Mcmillan. 1975; 189–192.
Andersson L., Letho V, Stenman S, Bodley RA, Virtamen I. Diazepam induces mitotic arrest at prometaphase by inhibiting centriolar separation. Nature 1981; 291: 247–248.
Lafi A, Parry JM. A study of the induction of aneuploidy and chromosome aberrations after diazepam, medazepam, midazolam and bromazepam treatment. Mutagenesis 1988; 3: 23–27.
Antoccia A, Degrassi F, Battistoni A, Ciliutti P, Tanzarella C. In vitro micronucleus test with kinetochore staining: Evaluation of test performance. Mutagenesis 1991; 6: 319–324.
Sbrana I, Di Sibio A, Lomi A, Scarcelli V. C-mitosis and numerical chromosome aberration analyses in human lymphocytes: 10 known or suspected spindle poisons. Mutat Res 1993; 287: 57–70.
Izzo M, Antoccia A, Degrassi F, Tanzarella C. Immunofluorescence analysis of diazepam-induced mitotic apparatus anomalies and chromosome loss in Chinese hamster cells. Mutagenesis 1998; 13: 445–451.
Cossarizza A, Baccarani-Contri M, Kalashnikova G, Franceschi C. (1993). A new method for the cytofluorimetric analysis of mitochondrial membrane potential using the J-aggregate forming lipophilic cation 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1). Biochem Biophys Res Commun 197: 40–45.
Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res 1983; 11: 1475–1489.
Doxsey S. Re-evaluating centrosome function. Nat Rev Mol Cell Biol 2001; 2: 688–698.
Lane HA, Nigg EA. Antibody microinjection reveals an essential role for human polo-like kinase 1 (Plk1) in the functional maturation of mitotic centrosomes. J Cell Biol 1996; 135: 1701–1713.
Shackelford RE, Kaufmann WK, Paules RS. Cell cycle control checkpoint mechanism and genotoxic stress. Environ Health Persp 1999; 107: 5–11.
Woods JA, Hadfield JA, Pettit GR, Fox BW, McGown AT. (1995). The interaction with tubulin of a series of stilbenes based on combretastatin A-4. Br J Cancer 71: 705–711.
Wassmann K, Benezra R. Mitotic checkpoints: From yeast to cancer. Curr Opin Genet Dev 2001; 11: 83–90.
Wassmann K, Liberal V, Benezra R. Mad2 phosphorylation regulates its association with Mad1 and the APC/C. EMBO J 2003; 22: 797–806.
Mayer TU, Kapoor TM, Haggarty SJ, King RW, Schreiber SL, Mitchison TJ. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science 1999; 286: 971–974.
Lens SM, Wolthuis RM, Klompmaker R, et al. Kauw J, Agami R, Brummelkamp T, Kops G, Medema RH. Survivin is required for a sustained spindle checkpoint arrest in response to lack of tension. EMBO J 2003; 22: 2934–2947.
Blajeski AL, Phan VA, Kottke TJ, Kaufmann SH. G(1) and G(2) cell-cycle arrest following microtubule depolymerization in human breast cancer cells. J Clin Invest 2002; 110: 91–99.
Masuda A, Maeno K, Nakagawa T, Saito H, Takahashi T. Association between mitotic spindle checkpoint impairment and susceptibility to the induction of apoptosis by anti-microtubule agents in human lung cancers. Am J Pathol 2003; 163: 1109–1116.
Bhalla KN Microtubule-targeted anticancer agents and apoptosis. Oncogene 2003; 22: 9075–9086.
Castedo M, Perfettini JL,Roumier T, et al. Mitotic catastrophe constitutes a special case of apoptosis whose suppression entails aneuploidy. Oncogene 2004; 23: 4362–4370.
Casellas P, Galiegue S, Basile AS. Peripheral benzodiazepine receptors and mitochondrial function. Neurochem Int 2002; 40: 475–486.
Patterson SD, Spahr CS, Daugas E, et al. Mass spectrometric identification of proteins released from mitochondria undergoing permeability transition. Cell Death Differ 2000; 7: 137–144.
Miccoli L, Poirson-Bichat F, et al. Potentiation of lonidamine and diazepam, two agents acting on mitochondria, in human glioblastoma treatment. J Natl Cancer Inst 1998; 90: 1400–1406.
Decaudin D, Castedo M, Nemati F, et al. Peripheral benzodiazepine receptor ligands reverse apoptosis resistance of cancer cells in vitro and in vivo. Cancer Res 2002; 62: 1388–1393.
Kleinerman RA, Brinton LA, Hoover R, Fraumeni JF Jr. Diazepam use and progression of breast cancer. Cancer Res 1984; 44: 1223–1225.
Costantini P, Jacotot E, Decaudin D, Kroemer G. Mitochondrion as a novel target of anticancer chemotherapy. J Natl Cancer Inst 2000; 92: 1042–1053.
Guicciardi ME, Leist M, Gores GJ. Lysosomes in cell death. Oncogene 2004; 23: 2881–2890.
Cregan SP, Dawson VL, Slack RS. Role of AIF in caspase-dependent and caspase-independent cell death. Oncogene 2004; 23: 2785–2796.
Janicke RU, Sprengart ML, Wati MR, Porter AG. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem 1998; 273: 9357–9360.
Chelli B, Lena A, Vanacore R, et al. Peripheral benzodiazepine receptor ligands: Mitochondrial transmembrane potential depolarization and apoptosis induction in rat C6 glioma cells. Bioch Pharmacol 2004; 68: 125–134.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vitale, I., Antoccia, A., Crateri, P. et al. Caspase-independent apoptosis is activated by diazepam-induced mitotic failure in HeLa cells, but not in human primary fibroblasts. Apoptosis 10, 909–920 (2005). https://doi.org/10.1007/s10495-005-2948-y
Issue Date:
DOI: https://doi.org/10.1007/s10495-005-2948-y