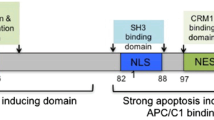
In the early 1990s it was discovered that the VP3/Apoptin protein encoded by the Chicken Anemia virus (CAV) possesses an inherent ability to specifically kill cancer cells. Apoptin was found to be located in the cytoplasm of normal cells while in tumor cells it was localized mainly in the nucleus.1 These differences in the localization pattern were suggested to be the main mechanism by which normal cells show resistance to Apoptin-mediated cell killing. Although the mechanism of action of Apoptin is presently unknown, it seems to function by the induction of programmed cell death (PCD) after translocation from the cytoplasm to the nucleus and arresting the cell cycle at G2/M, possibly by interfering with the cyclosome.2 In addition, cancer specific phosphorylation of Threonine residue 108 has been suggested to be important for Apoptin’s function to kill tumor cells.3 In contrast to the large number of publications reporting that nuclear localization, induction of PCD and phosphorylation of Apoptin is restricted to cancer cells, several recent studies have shown that Apoptin has the ability to migrate to the nucleus and induce PCD in some of the normal cell lines tested. There is evidence that high protein expression levels as well as the cellular growth rate may influence Apoptin’s ability to specifically kill tumor cells. Thus far both in vitro and in vivo studies indicate that Apoptin is a powerful apoptosis inducing protein with a promising prospective utility in cancer therapy. However, here we show that several recent findings contradict some of the earlier results on the tumor specificity of Apoptin, thus creating some controversy in the field. The aim of this article is to review the available data, some published and some unpublished, which either agree or contradict the reported “black and white” tumor cell specificity of Apoptin. Understanding what factors appear to influence its function should help to develop Apoptin into a potent anti-cancer agent.
Similar content being viewed by others
References
Danen-Van Oorschot AA, Fischer DF, Grimbergen JM, et al. Apoptin induces apoptosis in human transformed and malignant cells but not in normal cells. Proc Natl Acad Sci USA 1997; 94: 5843–5847.
Teodoro JG, Heilman DW, Parker AE, Green MR. The viral protein Apoptin associates with the anaphase-promoting complex to induce G2/M arrest and apoptosis in the absence of p53. Genes Dev 2004; 18: 1952–1957.
Rohn JL, Zhang YH, Aalbers RI, et al. A Tumor-specific Kinase Activity Regulates the Viral Death Protein Apoptin. J Biol Chem 2002; 277: 50820–50827.
Koch G, van Roozelaar DJ, Verschueren CA, van der Eb AJ, Noteborn MH. Immunogenic and protective properties of chicken anaemia virus proteins expressed by baculovirus. Vaccine 1995; 13: 763–770.
Noteborn MH, de Boer GF, van Roozelaar DJ, et al. Characterization of cloned chicken anemia virus DNA that contains all elements for the infectious replication cycle. J Virol 1991; 65: 3131–3139.
Noteborn MH, Todd D, Verschueren CA, et al. A single chicken anemia virus protein induces apoptosis. J Virol 1994; 68: 346–351.
Todd D, Creelan JL, Mackie DP, Rixon F, McNulty MS. Purification and biochemical characterization of chicken anaemia agent. J Gen Virol 1990; 71(Pt 4): 819–823.
Zhuang SM, Landegent JE, Verschueren CA, et al. Apoptin, a protein encoded by chicken anemia virus, induces cell death in various human hematologic malignant cells in vitro. Leukemia 1995; 9 Suppl 1: S118–120.
Zhuang SM, Shvarts A, van Ormondt H, et al. Apoptin, a protein derived from chicken anemia virus, induces p53-independent apoptosis in human osteosarcoma cells. Cancer Res 1995; 55: 486–489.
Noteborn MH, Zhang YH, van der Eb AJ. Apoptin specifically causes apoptosis in tumor cells and after UV-treatment in untransformed cells from cancer-prone individuals: A review. Mutat Res 1998; 400: 447–455.
Zhuang SM, Shvarts A, Jochemsen AG, et al. Differential sensitivity to Ad5 E1B-21kD and Bcl-2 proteins of apoptin-induced versus p53-induced apoptosis. Carcinogenesis 1995; 16: 2939–2944.
Danen-Van Oorschot AA, Zhang YH, Leliveld SR, et al. Importance of nuclear localization of apoptin for tumor-specific induction of apoptosis. J Biol Chem 2003; 278: 27729–27736.
Guelen L, Paterson H, Gaken J, et al. TAT-apoptin is efficiently delivered and induces apoptosis in cancer cells. Oncogene 2004; 23: 1153–1165.
Leliveld SR, Noteborn MH, Abrahams JP. Prevalent conformations and subunit exchange in the biologically active apoptin protein multimer. Eur J Biochem 2003; 270: 3619–3627.
Leliveld SR, Zhang YH, Rohn JL, Noteborn MH, Abrahams JP. Apoptin induces tumor-specific apoptosis as a globular multimer. J Biol Chem 2003; 278: 9042–9051.
Zhang YH, Leliveld SR, Kooistra K, et al. Recombinant Apoptin multimers kill tumor cells but are nontoxic and epitope-shielded in a normal-cell-specific fashion. Exp Cell Res 2003; 289: 36–46.
Cheng CM, Huang S, Chang YF, Chung WY, Yuo CY. The viral death protein Apoptin interacts with Hippi, the protein interactor of Huntingtin-interacting protein 1. Biochem Biophys Res Commun 2003; 305: 359–364.
Danen-van Oorschot AA, Voskamp P, Seelen MC, et al. Human death effector domain-associated factor interacts with the viral apoptosis agonist Apoptin and exerts tumor-preferential cell killing. Cell Death Differ 2004; 11: 564–573.
Zheng L, Schickling O, Peter ME, Lenardo MJ. The death effector domain-associated factor plays distinct regulatory roles in the nucleus and cytoplasm. J Biol Chem 2001; 276: 31945–31952.
Gervais FG, Singaraja R, Xanthoudakis S, et al. Recruitment and activation of caspase-8 by the Huntingtin-interacting protein Hip-1 and a novel partner Hippi. Nat Cell Biol 2002; 4: 95–105.
Kabra NH, Kang C, Hsing LC, Zhang J, Winoto A. T cell-specific FADD-deficient mice: FADD is required for early T cell development. Proc Natl Acad Sci USA 2001; 98: 6307–6312.
Yeh WC, Pompa JL, McCurrach ME, et al. FADD: Essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science 1998; 279: 1954–1958.
Chinnaiyan AM, O’Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 1995; 81: 505–512.
Ruland J, Duncan GS, Elia A, et al. Bc l10 is a positive regulator of antigen receptor-induced activation of NF-kappa B and neural tube closure. Cell 2001; 104: 33–42.
Ryan KM, O’Prey J, Vousden KH. Loss of nuclear factor-kappaB is tumor promoting but does not substitute for loss of p53. Cancer Res 2004; 64: 4415–4418.
Fujioka S, Schmidt C, Sclabas GM, et al. Stabilization of p53 is a novel mechanism for proapoptotic function of NF-kappaB. J Biol Chem 2004; 279: 27549–27559.
Kreuz S, Siegmund D, Rumpf JJ, et al. NFkappaB activation by Fas is mediated through FADD, caspase-8, and RIP and is inhibited by FLIP. J Cell Biol 2004; 166: 369–380.
Danen-van Oorschot AA, van Der Eb AJ, Noteborn MH. The chicken anemia virus-derived protein apoptin requires activation of caspases for induction of apoptosis in human tumor cells. J Virol 2000; 74: 7072–7078.
Janicke RU, Sprengart ML, Wati MR, Porter AG. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem 1998; 273: 9357–9360.
Mannick JB, Hausladen A, Liu L, et al. Fas-induced caspase denitrosylation. Science 1999; 284: 651–654.
Li SH, Lam S, Cheng AL, Li XJ. Intranuclear huntingtin increases the expression of caspase-1 and induces apoptosis. Hum Mol Genet 2000; 9: 2859–2867.
Li P, Nijhawan D, Budihardjo I, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997; 91: 479–489.
Zhang YH, Kooistra K, Pietersen A, Rohn JL, Noteborn MH. Activation of the tumor-specific death effector apoptin and its kinase by an N-terminal determinant of simian virus 40 large T antigen. J Virol 2004; 78: 9965–9976.
Wadia JS, Wagner MV, Ezhevsky SA, Dowdy SF. Apoptin/VP3 contains a concentration-dependent nuclear localization signal (NLS), not a tumorigenic selective NLS. J Virol 2004; 78: 6077–6078.
Pietersen AM, van der Eb MM, Rademaker HJ, et al. Specific tumor-cell killing with adenovirus vectors containing the apoptin gene. Gene Therapy 1999; 6: 882–892.
Rohn JL, Noteborn MH. The viral death effector Apoptin reveals tumor-specific processes. Apoptosis 2004; 9: 315–322.
Wang S, El-Deiry WS. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene 2003; 22: 8628–8633.
Saeki T, Mhashilkar A, Chada S, et al. Tumor-suppressive effects by adenovirus-mediated mda-7 gene transfer in non-small cell lung cancer cell in vitro. Gene Ther 2000; 7: 2051–2057.
Saeki T, Mhashilkar A, Swanson X, et al. Inhibition of human lung cancer growth following adenovirus-mediated mda-7 gene expression in vivo. Oncogene 2002; 21: 4558–4566.
Noteborn MH. Chicken anemia virus induced apoptosis: Underlying molecular mechanisms. Vet Microbiol 2004; 98: 89–94.
Schwarze SR, Hruska KA, Dowdy SF. Protein transduction: unrestricted delivery into all cells? Trends Cell Biol 2000; 10: 290–295.
Guelen L. Development of an efficient and tumour specific apoptosis inducing system using viral gene products. PhD thesis 2004, University of London.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tavassoli, M., Guelen, L., Luxon, B.A. et al. Apoptin: Specific killer of tumor cells?. Apoptosis 10, 717–724 (2005). https://doi.org/10.1007/s10495-005-0930-3
Issue Date:
DOI: https://doi.org/10.1007/s10495-005-0930-3