Abstract
The predacious mite Amblyseius swirskii Athias-Henriot is used as a biological control agent against various pests in greenhouses. Pollen offered as supplementary food is reported to improve their fast establishment and performance. However, the nutritional suitability of different pollens for A. swirskii is not sufficiently known yet. Pollens of 21 plant species were offered to the mites as exclusive food during preimaginal development. Preimaginal mortality and developmental time have been assessed, followed by a life-table analysis of the emerged adults and a calculation of demographic parameters. Amblyseius swirskii can feed exclusively on pollen, but the nutritional value of the pollens differed significantly. Pollens of Lilium martagon and Hippeastrum sp. were toxic, causing 100 % preimaginal mortality, probably due to secondary plant compounds. Hibiscus syriacus pollen was absolutely incompatible for the juvenile and adult mites, possibly due to their external morphology, differing from all the other pollens tested and leading to 100 % preimaginal mortality also. Considering all parameters, feeding on Aesculus hippocastanum, Crocus vernus, Echinocereus sp. and Paulownia tomentosa pollens lead to the best performance of the mites. Feeding on most pollens resulted in no or low preimaginal mortality of A. swirskii, but affected significantly developmental time, adult longevity, and reproduction parameters. Commercial bee pollen was not able to improve life-table parameters compared to pure pollen of the plant species. Pollens of Helianthus annuus, Corylus avellana and a Poaceae mix were less suitable as food source and resulted in a poor performance of all tested parameters. Compared with literature data, 18 pollens tested proved to be a similar or better food source than cattail pollen, qualifying A. swirskii as a positively omnivorous type IV species. Pollens of Ricinus communis and Zea mays can be recommended as supplementary food offered as banker plants, and A. hippocastanum and Betula pendula pollen is recommended to be used as dispersible pollen in greenhouses.
Graphical Abstract
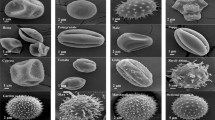
Pollen grains after feeding by A. swirskii females (a—Hibiscus; b—Horse chestnut; c—Narcissus; d—Ricinus; e—Tulip; f—A. swirskii chelicers).



Similar content being viewed by others
References
Abdallah AA, Zhang ZQ, Masters GJ, McNeill S (2001) Euseius finlandicus (Acari: Phytoseiidae) as a potential biocontrol agent against Tetranychus urticae (Acari: Tetranychidae): life history and feeding habits on three different types of food. Exp Appl Acarol 25:833–847
Adar E, Inbar M, Gal S, Doron N, Zhang ZQ, Palevsky E (2012) Plant-feeding and non-plant feeding phytoseiids: differences in behavior and cheliceral morphology. Exp Appl Acarol 58:341–357
Addison JA, Hardman JMJ, Walde SJ (2000) Pollen availability for predaceous mites on apple: spatial and temporal heterogeneity. Exp Appl Acarol 24:1–18
Adler LS (2000) The ecological significance of toxic nectar. Oikos 91:409–420
Adler LS, Wink M (2001) Transfer of quinolizidine alkaloids from hosts to hemiparasites in two Castilleja-Lupinus associations: analysis of floral and vegetative tissues. Biochem Syst Ecol 29:551–561
Al-Shammery KA (2011) Plant pollen as an alternative food source for rearing Euseius scutalis (Acari: Phytoseiidae) in Hail, Saudi Arabia. J Entomol 8:365–374
Antal DS (2010) Medicinal plants with antioxidant properties from Banat region (Romania): a rich pool for the discovery of multi-target phytochemicals active in free-radical related disorders. Analele Universitatii din Oradea-Fascicula Biology 22:14–22
Arthurs S, McKenzie CL, Chen J, Dogramaci M, Brenna M, Houben K, Osborne L (2009) Evaluation of Neoseiulus cucumeris and Amblyseius swirskii (Acari: Phytoseiidae) as biological control agents of chilli thrips, Scirtothrips dorsalis (Thysanoptera: Thripidae) on pepper. Biol Control 49:91–96
Atrouse OM, Oran OM, Al-Abbadi SY (2004) Chemical analysis and identification of pollen grains from different Jordanian honey samples. Int J Food Sci Technol 39:413–417
Baker HG, Baker I (1975) Studies of nectar-constitution and pollinator-plant coevolution. In: Gilbert LE, Raven PH (eds) Coevolution of plants and animals. University of Texas Press, Austin, pp 100–140
Baker HG, Baker I (1979) Starch in angiosperm pollen grains and its evolutionary significance. Am J Bot 66:591–600
Bermúdez P, Vargas R, Cardemil A, López E (2010) Effect of pollen from different plant species on development of Typhlodromus pyri (Scheuten) (Acari: Phytoseiidae). Chil J Agric Res 70:408–416
Beug HJ (2004) Leitfaden der Pollenbestimmung für Mitteleuropa und angrenzende Gebiete. Verlag Dr. Friedrich Pfeil, Munich
Bibi N, Manzoor H, Naveed A (2008) Palynological study of some cultivated species of Genus Hibiscus from west frontier province (N.W.F.P.) Pakistan Noreen. Pak J Bot 40:1561–1569
Bibi N, Naveed A, Manzoor H, Ajab KM (2010) Systematic implications of pollen morphology in the family Malvaceae from north west frontier province, Pakistan. Pak J Bot 42:2205–2214
Bogdanov S (2006) Contaminants of bee products. Apidologie 37:1–18
Brodbeck BV, Stavisky J, Funderburk JE, Andersen PC, Olson SM (2001) Flower nitrogen status and population of Frankliniella occidentalis feeding on Lycopersicon esculentum. Entomol Exp Appl 99:165–172
Broufas GD, Koveos DS (2000) Effect of different pollens on development, survivorship and reproduction of Euseius finlandicus (Acari: Phytoseiidae). Environ Entomol 29:743–749
Castagnoli M, Simoni S (1999) Effect of long-term feeding history on functional and numerical response of Neoseiulus californicus (Acari: Phytoseiidae). Exp Appl Acarol 23:217–234
Ceska O, Styles ED (1984) Flavonoids from Zea mays pollen. Phytochemistry 23:1822–1824
Chantarudee A, Phuwapraisirisan P, Kimura K, Okuyama H, Mori H, Kimura A, Chanchao C (2012) Chemical constituents and free radical scavenging activity of corn pollen collected from Apis mellifera hives compared to floral corn pollen at Nan, Thailand. BMC Complem Altern M 12:45
Coll M, Guershon M (2002) Omnivory in terrestrial arthropods: mixing plant and prey diets. Annu Rev Entomol 47:267–297
Cooper MR, Johnson AW (1984) Poisonous plants in Britain and their effects on animals and man. Her Majesty’s Stationery Office, London 305
Croft BA, Blackwood JS, McMurtry JA (2004) Classifying life-style types of phytoseiid mites: diagnostic traits. Exp Appl Acarol 33:247–260
da Silva CV, de Mesquita LX, Maracajá PB, Soto-Blanco B (2010) Toxicity of Mimosa tenuiflora pollen to Africanized honey bees (Apis mellifera L.). Acta Sci Vet 38:161–163
Dabija T (2010) Study of amino acids in pollen’s composition. Bull UASVM Anim Sci Biol 67:1–2
Danks HV (2006) Short life cycles in insects and mites. Can Entomol 138:407–463
de Moraes GJ, McMurtry JA, Denmark HA, Campos CB (2004) A revised catalogue of the mite family Phytoseiidae. Zootaxa 434:1–494
Detzel A, Wink M (1993) Attraction, deterrence or intoxication of bees (Apis mellifera) by plant allelochemicals. Chemoecology 4:8–18
Dobson HEM, Bergström G (2000) The ecology and evolution of pollen odors. Plant Syst Evol 222:63–87
Duso C, Malagnini V, Paganelli A, Aldegheri L, Bottini M, Otto S (2004) Pollen availability and abundance of predatory phytoseiid mites on natural and secondary hedgerows. Biocontrol 49:397–415
Elsawi SA, Abou-Awad BA (1992) Starvation and fertilization affecting reproduction in Amblyseius swirskii Athias-Henriot and A. gossypi El-Badry (Acari, Phytoseiidae). J Appl Entomol 113:239–243
Engel R (1991) Der Einfluß von Ersatznahrung, Wirtspflanze und Mikroklima auf das System Typhlodromus pyri Scheuten (Acari, Phytoseiidae): Panonychus ulmi Koch (Acari, Tetranychidae) im Weinbau. Dissertation, University of Hohenheim
Engel R, Ohnesorge B (1994) The role of alternative food and microclimate in the system Typhlodromus pyri Scheuten (Acari, Phytoseiidae) and Panonychus ulmi Koch (Acari, Tetranychidae) on grapevines. I. Laboratory investigations. J Appl Entomol 118:129–150
Faeth SH, Saari S (2012) Fungal grass endophytes and arthropod communities: lessons from plant defence theory and multitrophic interactions. Fungal Ecol 5:364–371
Flechtmann CHW, McMurtry JA (1992a) Studies on how phytoseiid mites feed on spider mites and pollen. Int J Acarol 18:157–162
Flechtmann CHW, McMurtry JA (1992b) Studies of cheliceral and deutosternal morphology of some Phytoseiidae (Acari: Mesostigmata) by scanning electron microscopy. Int J Acarol 18:163–169
Fu JH, Lei LG, Chen LB, Qiu GZ (2001) Wall ultrastructure and cytochemistry and the longevity of pollen of three grass species. Aust J Bot 49:771–776
Fuller TC, McClintock E (1986) Poisonous plants of California. University of California Press, Berkeley
Gerson U, Weintraub PG (2007) Mites for control of pests in protected cultivation. Pest Manag Sci 63:658–676
Gerson U, Weintraub PG (2012) Mites (Acari) as a factor in greenhouse management. Annu Rev Entomol 57:229–247
Gerson U, Smiley L, Ochoa R (2003) Mites (Acari) for pest control. Blackwell, Oxford
Gnanvossou D, Hanna R, Yaninek JS, Toko M (2005) Comparative life history traits of three neotropical phytoseiid mites maintained on plant-based diets. Biol Control 35:32–39
González-Fernández JJ, De La Peña F, Hormaza JI, Boyero JR, Vela JM, Wong E, Trigo MM, Montserrat M (2009) Alternative food improves the combined effect of an omnivore and a predator on biological pest control. A case study in avocado orchards. B Entomol Res 99:433–444
Gotoh T, Yamaguchi K, Mori K (2004) Effect of temperature on life history of the predatory mite Amblyseius californicus (Acari: Phytoseiidae). Exp Appl Acarol 32:15–30
Grout TG, Richards GI (1992) The dietary effect of windbreak pollens on longevity and fecundity of a predacious mite Euseius addoensis (Acari: Phytoseiidae) found in citrus orchards in South Africa. B Entomol Res 82:317–320
Hagedorn HH (1968) Effect of the age of pollen used in pollen supplements on their nutritive value for the honeybee. I. Effect on thoracic weight, development of hypopharyngeal glands and brood rearing. J Apic Res 7:89–95
Hanley ME, Franco M, Pichon S, Darvill B, Goulson D (2008) Breeding system, pollinator choice and variation in pollen quality in British herbaceous plants. Funct Ecol 22:592–598
Herbert EW, Shimanuki H (1978) Chemical composition and nutritive value of bee collected and bee stored pollen. Apidologie 9:33–40
Hernandez-Suarez E, Velasquez MC, Paz-Gonzalez MI, Gonzalez FJ, Carnero A, Ferragut FJ (2006) Effect of different types of prey on fecundity of the phytoseiid mite Typhlodromips swirskii, a potential biological control agent for horticultural greenhouse pests in the Canary Islands. Bull OILB/SROP 29:137
Hoogerbrugge H, van Houten Y, van Baal E, Bolckmans K (2008) Alternative food sources to enable establishment of Amblyseius swirskii (Athias-Henriot) on chrysanthemum without pest presence. Bull OILB/SROP 32:79–82
Huang N, Enkegaard A, Osborne LS, Ramakers PMJ, Messelink GJ, Pijnakker J, Murphy G (2011) The banker plant method in biological control. Crit Rev Plant Sci 30:259–278
Hulshof J, Vanninen I (2002) The Western Flower Thrips Frankliniella occidentalis feeding on pollen and the implications for its control. Thrips and tospoviruses. Proceedings of the seventh international symposium on thysanoptera, Reggio Calabria, Italy 173–179
Hulshof J, Ketoja E, Vanninen I (2003) Life history characteristics of Frankliniella occidentalis on cucumber leaves with and without supplemental food. Entomol Exp Appl 108:19–32
Işik S, Dönmez EO (2006) Pollen morphology of some Turkish Crocus L. (Iridaceae) species. Acta Biol Crac Bot 48:85–91
James DG (1989) Influence of diet on development, survival and oviposition in an Australian phytoseiid, Amblyseius victoriensis (Acari: Phytoseiidae). Exp Appl Acarol 6:1–10
Kasap I (2005) Life-history traits of the predaceous mite Kampimodromus aberrans (Oudemans) (Acarina: Phytoseiidae) on four different types of food. Biol Control 35:40–45
Klimko M, Kluza M, Kreft A (2000) Morphology of pollen grains in three varieties of Helianthus annuus L. Rocz AR Pozn CCCXXII Bot 3:135–142
Kolokytha PD, Fantinou AA, Papadoulis GT (2011) Effect of several different pollens on the bio-ecological parameters of the predatory mite Typhlodromus athenas Swirski and Ragusa (Acari: Phytoseiidae). Environ Entomol 40:597–604
Kreiter S, Tixier MS, Croft BA, Auger P, Barret D (2002) Plants and leaf characteristics influencing the predaceous mite Kampimodromus aberrans (Acari: Phytoseiidae) in habitats surrounding vineyards. Environ Entomol 31:648–660
Kurtz EB (1948) Pollen grain characters of certain Cactaceae. Bull Torrey Bot Club 75:516–522
Lampe KF, McCann MA (1985) AMA Handbook of poisonous and injurious plants. American Medical Association, Chicago
Lee H, Gillespie DR (2011) Life tables and development of Amblyseius swirskii (Acari: Phytoseiidae) at different temperatures. Entomol Exp Appl 53:17–27
Linskens HF, Pfahler PL (1977) Genotypic effects on the amino acid relationships in maize (Zea mays L.) pollen and style. Theor Appl Genet 50:173–177
Loper GM, Cohen AC (1987) Amino acid content of dandelion pollen, a honey bee (Hymenoptera: Apidae) nutritional evaluation. J Econ Entomol 80:14–17
Luh HK, Croft BA (2001) Quantitative classification of life-style types in predaceous phytoseiid mites. Exp Appl Acarol 25:403–424
Manning R (2001) Fatty acids in pollen: a review of their importance for honeybees. Bee World 82:60–75
Mansour F (1990) Species presence and density of tetranychid and phytoseiid mites in unsprayed and sprayed apple orchards in northern Israel. Phytoparasitica 18:135–141
Matsuo T, Mochizuki M, Yara K, Mitsunaga T, Mochizuki A (2003) Suitability of Pollen as an alternative diet for Amblyseius cucumeris (Oudeman). Jpn J Appl Entomol Z 47:153–158
McMurtry JA, Croft BA (1997) Life-styles of Phytoseiid mites and their roles in biological control. Annu Rev Entomol 42:291–321
McMurtry JA, Rodrigues JG (1987) Nutritional ecology of phytoseiid mites. In: Slanski F, Rodrigues JG (eds) Nutritional ecology of insects, mites, spiders and related invertebrates. Wiley Interscience, New York, pp 609–644
Messelink GJ, van Steenpaal SEF, van Wensveen W (2005) Typhlodromips swirskii (Athias-Henriot) (Acari: Phytoseiidae): a new predator for thrips control in greenhouse cucumber. Bull SROP/WPRS 28:183–186
Messelink GJ, van Maanen R, van Steenpaal SEF, Janssen A (2008) Biological control of thrips and whiteflies by a shared predator: two pests are better than one. Biol Control 44:372–379
Momen FM (2004) Suitability of the pollen grains, Ricinus communis and Helianthus annuus as food for six species of phytoseiid mites (Acari: Phytoseiidae). Acta Phytopathol Entomol Hung 39:415–422
Momen F (2009) Potential of three species of predatory phytoseiid mites as biological control agents of the peach silver mite, Aculus fockeui (Acari: Phytoseiidae and Eriophyidae). Acta Phytopathol Entomol Hung 44:151–158
Momen FM (2011) Life tables and feeding habits of Proprioseiopsis cabonus, a specific predator of tydeid mites (Acari: Phytoseiidae and Tydeidae). Acarina 19:103–109
Momen FM, Abdel-Khalek A (2008) Effect of the tomato rust mite Aculops lycopersici (Acari: Eriophyidae) on the development and reproduction of three predatory phytoseiid mites. Int J Trop Insect Sci 28:53–57
Momen FM, El-Saway SA (1993) Biology and feeding behaviour of the predatory mite, Amblyseius swirskii (Acari: Phytoseiidae). Acarologia 34:199–204
Mullin CA, Alfatafta AA, Harman JL, Everett SL, Serino AA (1991) Feeding and toxic effects of floral sesquiterpene lactones, diterpenes, and phenolics from sunflower (Helianthus annuus L.) on Western Corn Rootworm. J Agr Food Chem 39:2293–2299
Negloh K, Hanna R, Schausberger P (2008) Comparative demography and diet breadth of Brazilian and African populations of the predatory mite Neoseiulus baraki, a candidate for biological control of coconut mite. Biol Control 46:523–531
Nepi M, Cresti L, Guarnieri M, Pacini E (2010) Effect of relative humidity on water content, viability and carbohydrate profile of Petunia hybrid and Cucurbita pepo pollen. Plant Syst Evol 284:57–64
Nguyen TV, Shih CT (2010) Development of Neoseiulus womersleyi (Schicha) and Euseius ovalis (Evanz) feeding on four tetranychid mites (Acari: Phytoseiidae, Tetranychidae) and pollen. J Asia Pac Entomol 13:289–296
Nichols CI, Altieri MA (2004) The agroecological engineering: for pest management. In: Gurr G, Wratten S, Altieri M (eds) Ecological engineering for pest management. CSIRO Publishing, Collingwood, pp 33–54
Nicolson SW (2011) Bee food: the chemistry and nutritional value of nectar, pollen and mixtures of the two. Afr Zool 46:197–204
Nomikou M, Janssen A, Schraag R, Sabelis MW (2001) Phytoseiid predators as potential biological control agents for Bemisia tabaci. Exp Appl Acarol 25:271–291
Nomikou M, Janssen A, Schraag R, Sabelis MW (2002) Phytoseiid predators suppress populations of Bemisia tabaci on cucumber plants with alternative food. Exp Appl Acarol 27:57–68
Nomikou M, Janssen A, Sabelis MW (2003) Phytoseiid predators of whiteflies feed and reproduce on non-prey food sources. Exp Appl Acarol 31:15–26
Nomikou M, Sabelis MW, Janssen A (2010) Pollen subsidies promote whitefly control through the numerical response of predatory mites. Biocontrol 55:253–260
Onzo A, Houedokoho AF, Hanna R (2011) Potential of the predatory mite, Amblyseius swirskii to suppress the broad mite, Polyphagotarsonemus latus on the gboma eggplant, Solanum macrocarpon. J Insect Sci 12:7
Ouyang Y, Grafton-Cardwell E, Bugg RL (1992) Effects of various pollens on development, survivorship, and reproduction of Euseius tularensis (Acari: Phytoseiidae). Environ Entomol 21:1371–1376
Overmeer WPJ (1985) Rearing and handling. In: Helle W, Sabelis MW (eds) Spider mites, vol 1B. Elsevier, Amsterdam, pp 161–170
Pacini E, Guarnieri M, Nepi M (2006) Pollen carbohydrates and water content during development, presentation, and dispersal: a short review. Protoplasma 228:73–77
Palevsky E, Gal S, Maoz Y, Abrahams Y, Melamed E, Domeratzky S, Gross S, Shmueli S, Gan-Mor S, Ronen B, Argov Y (2010) Windborne pollen provisioning cover crops (WPPCC) for enhancing the population levels of Euseius scutalis and improving citrus rust mite control. Bull SROP/WPRS 62:93–97
Park HH, Shipp L, Buitenhuis R, Ahn JJ (2011) Life history parameters of a commercially available Amblyseius swirskii (Acari: Phytoseiidae) fed on cattail (Typha latifolia) pollen and tomato russet mite (Aculops lycopersici). J Asia Pac Entomol 14:497–501
Pernal SF, Currie RW (2000) Pollen quality of fresh and 1-year-old single pollen diets for worker honey bees (Apis mellifera L.). Apidologie 31:387–409
Pina T, Sá Argolo P, Urbaneja A, Jacas JA (2012) Effect of pollen quality on the efficacy of two different life-style predatory mites against Tetranychus urticae in citrus. Biol Control 61:176–183
Praz CJ, Müller A, Dorn S (2008) Specialzed bees fail to develop on non-host pollen: do plants chemically protect their pollen? Ecology 89:795–804
Rabie AL, Wells JD, Dent LK (1983) The nitrogen content of pollen protein. J Apic Res 22:119–123
Ragusa E, Tsolakis H, Jordà Palomero R (2009) Effect of pollens and preys on various biological parameters of the generalist mite Cydnodromus californicus. Bull Insectol 62:153–158
Ramakers PMJ, Voet SJP (1996) Introduction of Amblyseius degenerans for thrips control in sweet peppers with potted castor beans as banker plants. Bull SROP/WPRS 19:127–130
Reynard GB, Norton JB (1942) Poisonous plants of Maryland in relation to livestock. Univ Md Agric Exp Stn A10:312
Roth L, Daunderer M, Kormann K (2008) Giftpflanzen-Pflanzengifte. 5th edn. Ecomed, Landsberg
Roulston TH, Buchmann SL (2000) A phylogenetic reconsideration of the pollen starch-pollination correlation. Evol Ecol Res 2:627–643
Roulston TH, Cane JH, Buchmann SL (2000) What governs the protein content of pollen grains: pollinator preferences, pollen-pistil interactions, or phylogeny? Ecol Monogr 70:617–643
Sabelis MW (1985) Sex allocation. In: Helle W, Sabelis MW (eds) Spider mites. Their biology, natural enemies and control, vol IB. Elsevier, Amsterdam, pp 83–94
Schausberger P, Croft BA (1999) Activity, feeding, and development among larvae of specialist and generalist phytoseiid mite species (Acari: Phytoseidae). Environ Entomol 28:322–329
Schausberger P, Walzer A (2001) Combined versus single species release of predaceous mites: predator–predator interactions and pest suppression. Biol Control 20:269–278
Schausberger P, Walzer A, Hoffmann D, Hasan R (2010) Food imprinting revisited: early learning in foraging predatory mites. Behaviour 147:883–897
Schmidt JO, Buchmann SL (1985) Pollen digestion and nitrogen utilization by Apis mellifera L. (Hymenoptera: Apidae). Comp Biochem Physiol A Mol Integr Physiol 82:499–503
Schulz-Langner E (1967) Über den Trachtwert der Rosskastanie (Aesculus hippocastanum) unter besonder Berücksichtigung des Saponin-Gehaltes im Nectar. Z Bienenforsch 9:49–65
Shaheen N, Khan MA, Hayat MQ, Yasmin G (2009) Pollen morphology of 14 species of Abutilon and Hibiscus of the family Malvaceae (sensu stricto). J Med Plants Res 3:921–929
Singh MB, Knox RB (1985) β-Galactosidases of Lilium pollen. Phytochemistry 24:1639–1643
Skirvin D, Kravar-Garde L, Reynolds K, Jones J, de Courcy Williams M (2006) The influence of pollen on combining predators to control Frankliniella occidentalis in ornamental chrysanthemum crops. Biocontrol Sci Technol 16:99–105
Somerville DC, Nicol HI (2006) Crude protein and amino acid composition of honey bee-collected pollen pellets from south-east Australia and a note on laboratory disparity. Aust J Exp Agric 46:141–149
Southwood TRE, Henderson PA (2000) Ecological methods. Blackwell, Oxford
Stanley RG, Linskens HG (1974) Pollen, biology, biochemistry and management. Springer, Berlin
Swirski E, Amitai S (1997) Annotated list of phytoseiid mites (Mesostigmata: Phytoseiidae) in Israel. Isr J Entomol 31:21–46
Swirski E, Amitai S, Dorzia N (1967) Laboratory studies on the feeding, development and oviposition of the predatory mites Amblyseius rubini swirskii and Amblyseius swirskii Athias(Acarina, Phytoseiidae) on variuos kinds of food substances. Isr J Agric Res 17:101–109
Takahashi M, Kouchi J (1988) Ontogenetic development of spinous exine in Hibiscus syriacus (Malvaceae). Am J Bot 75:1549–1558
Tasei JN, Aupinel P (2008) Nutritive value of 15 single pollens and pollen mixes tested on larvae produced by bumblebee workers (Bombus terrestris, Hymenoptera: Apidae). Apidologie 39:397–409
Todd FE, Bretherick O (1942) The composition of pollens. J Econ Entomol 3:312–317
Tuovinen T, Lindqvist I (2010) Maintenance of predatory phytoseiid mites for preventive control of strawberry tarsonemid mite Phytonemus pallidus in strawberry plant propagation. Biol Control 54:119–125
van Houten YM, Ostilie ML, Hoogerbrugge H, Bolckmans K (2005) Biological control of western flower thrips on sweet pepper using the predatory mites Amblyseius cucumeris, Iphiseius degenerans, A. andersoni and A. swirskii. Bull SROP/WPRS 28:283–286
van Rijn PCJ, Tanigoshi LK (1999a) Pollen as food for the predatory mites Iphiseius degenerans and Neoseiulus cucumeris (Acari: Phytoseiidae): dietary range and life history. Exp Appl Acarol 23:785–802
van Rijn PCJ, Tanigoshi LK (1999b) The contribution of extrafloral nectar to survival and reproduction of the predatory mite Iphiseius degenerans on Ricinus communis. Exp Appl Acarol 23:281–296
van Rijn PCJ, van Houten YM, Sabelis MW (2002) How plants benefit from providing food to predators even when it is also edible to herbivores. Ecology 83:2664–2679
Vantornhout I, Minnaer HL, Tirry L, de Clercq P (2005) Influence of diet on life table parameters of Iphiseius degenerans. Exp Appl Acarol 35:183–195
Villanueva RT, Childers CC (2004) Phytoseiidae increase with pollen deposition on citrus leaves. Fla Entomol 87:609–611
Vinson CG (1927) Some nitrogenous constituents of corn pollen. Division of Agricultural Biochemistry, University of Minnesota, Minneapolis
Weintraub PG, Kleitman S, Mori R, Gan-Mor S, Ganot L (2009) Novel application of pollen to augment the predator Amblyseius swirskii on greenhouse sweet pepper. Bull SROP/WPRS 50:119–124
Wermelinger B, Oertli JJ, Delucchi V (1985) Effect of host plant nitrogen fertilization on the biology of the two-spotted spider mite, Tetranychus urticae. Entomol Exp Appl 38:23–28
Wimmer D, Hoffmann D, Schausberger P (2008) Prey suitability of western flower thrips, Frankliniella occidentalis, and onion thrips, Thrips tabaci, for the predatory mite Amblyseius swirskii. Biocontrol Sci Technol 18:541–550
Yue B, Tsai JH (1996) Development, survivorship, and reproduction of Amblyseius largoensis (Acari: Phytoseiidae) on selected plant pollens and temperatures. Environ Entomol 25:488–494
Zannou ID, Hanna R (2011) Clarifying the identity of Amblyseius swirskii and Amblyseius rykei (Acari: Phytoseiidae): are they two distinct species or two populations of one species? Exp Appl Acarol 53:339–347
Acknowledgments
The authors are indebted to Sandra Gerken for her substantial contribution to SEM–photographs and to Elena Vogel (both at the Institute of Phytomedicine, University of Hohenheim, Stuttgart, Germany) for her help with mite breeding. We are also grateful to Erica Rücker and Bärbel Rassow (Institute of Botany, University of Hohenheim) for their expert help with the SEM-studies. This study was in part supported by a PhD grant from the European Commission Erasmus Mundus External Cooperation Window (IAMONET-RU). Our sincere thanks also go to the reviewers for their highly appreciated comments on the first version of our manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goleva, I., Zebitz, C.P.W. Suitability of different pollen as alternative food for the predatory mite Amblyseius swirskii (Acari, Phytoseiidae). Exp Appl Acarol 61, 259–283 (2013). https://doi.org/10.1007/s10493-013-9700-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-013-9700-z