Abstract
Bartonella species are fastidious, gram negative bacteria, some of which are transmitted by arthropod vectors, including fleas, sandflies, and lice. There is very little information regarding the interaction and/or transmission capabilities of Bartonella species by ticks. In the present study, we demonstrate successful infection of the Amblyomma americanum cell line, AAE12, by seven Bartonella isolates and three Candidatus Bartonella species by electron or light microscopy. With the exception of Bartonella bovis, infection with all other examined Bartonella species induced cytopathic effects characterized by heavy cellular vacuolization and eventually cell lysis. Furthermore, using quantitative real time PCR (qPCR), we demonstrated significant amplification of two B. henselae genotype I isolates in the A. americanum cell line over a 5 days period. Ultimately, tick-cell derived Bartonella antigens may prove useful for the development of more sensitive diagnostic reagents and may assist in the development of an effective vaccine to prevent the further spread of disease caused by these organisms.
Similar content being viewed by others
Introduction
Tick cell lines have been used for the propagation of various bacteria, viruses, and protozoans and have proved crucial in isolating several previously “unculturable” organisms, such as Borrelia lonestari (Varela et al. 2004; Bell-Sakyi et al. 2007). Morphologically, Anaplasma marginale grown within an Ixodes scapularis tick cell line (IDE8) appears similar to bacterial clusters visible within infected tick tissues, suggesting that tick cell lines may provide an in vitro means to study the biology of tick transmitted pathogens (Munderloh et al. 1996; Blouin and Kocan 1998). Because of their permissive quality, non-tick transmitted organisms have been cultured in tick cell lines, as well. Pornwiroon et al. (2006) isolated Rickettsia felis, bacteria transmitted by the cat flea, Ctenocephalides felis, within an I. scapularis tick cell line (ISE6) directly from laboratory reared adult fleas. Besides study of the interaction of an organism with an arthropod host in vitro, tick cell lines have also proved crucial for the development of diagnostic reagents and appear promising as a source of potential antigens for vaccine development (de la Fuente et al. 2002; Kocan et al. 2001; Saliki et al. 1998).
Bartonella species are fastidious, hemotropic, gram-negative bacteria associated with several recently recognized or re-emerging diseases. These organisms are able to infect a wide variety of mammalian hosts including humans, canines, felines, and livestock and appear to be transmitted by a diverse spectrum of arthropod vectors, including fleas, sandflies, lice, and potentially ticks and biting flies, as well as by direct transmission via bite/scratch from an infected host (reviewed in Billeter et al. 2008a). Recently, Tate et al. (2005) cultured a Bartonella sp., closely related to B. schoenbuchensis, within the I. scapularis cell line (ISE6) obtained from infected deer blood, however, the authors did not establish whether the bacteria grew intracellularly, extracellularly, or in both locations. As Bartonella DNA could not be detected by PCR in deer blood samples prior to the inoculation of these samples into tick cells, either biological components within the media or active replication within tick cells appears to be responsible for the authors’ results (Tate et al. 2005). We were interested in examining whether a tick cell line could serve in the maintenance and propagation of Bartonella species. Since 1992, it has been suggested that ticks may serve in the maintenance of Bartonella species (Lucey et al. 1992). Case studies and molecular/serological epidemiology surveys in humans and canines and the detection of Bartonella DNA in a variety of tick species throughout the world suggest ticks may be competent vectors of Bartonella species (Billeter et al. 2008a). Within the current study, we demonstrate the ability of several different Bartonella species to infect an Amblyomma americanum, AAE12, cell line. Furthermore, by using quantitative qPCR, we observed significant growth of two B. henselae, the agent of Cat Scratch disease, isolates in the presence of tick cells and in conditioned medium. To our knowledge, this is the first report describing the infection and replication of Bartonella species within a tick cell line.
Materials and methods
Tick cell culture
Amblyomma americanum (AAE12), I. scapularis (ISE6), and Rhipicephalus sanguineus (RSE8) embryonic cell lines were utilized to study the potential infection and growth of Bartonella species within tick cells (Kurtti et al. 1982; Munderloh et al. 1999; Bell-Sakyi et al. 2007). Uninfected and infected AAE12 and ISE6 were maintained at 34°C in sealed 25 cm2 flasks (Greiner Bio-One, Longwood, FL, USA) or in 24 well plates (Becton Dickinson, Franklin Lakes, NJ, USA) with L-15B300 medium (Munderloh et al. 1999) (L-15 medium diluted by 1/4 volume with cell culture water) and supplemented with 10% heat-inactivated fetal bovine serum (FBS) (HyClone, Logan, UT, USA), 5% tryptose phosphate broth (TPB) (Difco Laboratories, Detroit, MI, USA), and 0.1% bovine lipoprotein concentrate (BLC) (MP Biomedials, Irvine, CA, USA). Uninfected and infected RSE8 cells were maintained at 31°C. The RSE8 cell line does not require the addition of cell culture water to the L-15B medium, however, the concentration of supplemental reagents, FBS, TPB, and BLC, remained the same, and the pH of all media was adjusted to 7.5–7.6 with 10 N NaOH.
Bacterial strains and growth conditions
The Bartonella species, sources, and providers of isolates are summarized in Table 1. All isolates were grown at 35°C in a 5% CO2 atmosphere on trypticase soy agar (TSA) with 5% sheep blood (Becton Dickinson) for approximately 4–6 days prior to infection of the cell lines. Two B. henselae genotype I isolates, which will be referred to as B. henselae isolates A and B, were utilized for the growth kinetic studies within the AAE12 tick cell line. There are differences within the 16S–23S intergenic spacer region (ITS) between B. henselae isolate A (identical to GenBank # AJ441256) and B. henselae isolate B (identical to GenBank # BX897699.1), however, sequencing of the citrate synthase (gltA) and riboflavin synthase (ribC) genes revealed 100% homology between the two isolates. Isolate B has a 16S–23S ITS sequence that is identical to the Houston-1 isolate (ATCC 49882).
In order to compare the growth rates of transformed and non-transformed bacteria within a tick cell line, B. henselae isolates were transformed with a green fluorescent protein (GFP) plasmid (Fournier et al. 1999). The bacteria were transformed with the pJMB-GFP plasmid, a pBBR1-MCS2 with a 970 bp HindIII fragment containing the B. bacilliformis flagellin promoter (fla-pro) upstream of gfpmut3a, following the same conditions described by Fournier et al. (1999). The B. henselae strains expressing the green fluorescent protein will be referred to as B. henselae isolate A-GFP and B. henselae isolate B-GFP.
The transformed bacteria were selected using agar plates made with Bartonella alpha-Proteobacteria growth medium (BAPGM) containing 10% defribinated sheep (HemoStat Laboratories, Dixon, CA, USA) or rabbit (Becton Dickinson) blood with 40 μg/ml of kanamycin sulfate (MP Biomedicals) (Maggi et al. 2005). BAPGM-kanamycin plates were also placed at 35°C in a 5% CO2 atmosphere and bacteria were allowed to grow for 4–6 days prior to infection of tick cells.
Bartonella henselae growth kinetic studies
Twenty-four hours prior to infection, approximately 1.9–2.5 × 105 AAE12 cells per well (assessed by direct hemacytometer count) were seeded in 1 ml of L-15B300 growth medium into 15 wells of 24-well plates. Six 24-well plates were used per infection study. Each plate contained triplicate wells of non-infected cells and triplicate wells infected with parent strains of B. henselae A and B and B. henselae isolate A-GFP and B. henselae isolate B-GFP. Each growth kinetic study was performed in duplicate.
On the day of infection, medium was replaced in all wells with fresh L-15B300 medium or L-15B300 containing 40 μg/ml kanamycin sulfate. The non-transformed and GFP expressing B. henselae isolates were collected from agar plates using a sterile loop and resuspended in 3–5 ml of L-15B300 medium. Tick cells were inoculated with 50 μl from each of the bacterial resuspensions into designated triplicate wells (5.8 × 107–2.9 × 108 CFU/ml). To determine the viable cell count (colony forming units/ml) on the day of infection for each isolate, serial dilutions of the bacterial resuspensions were performed and 100 μl of the inoculum was added onto either TSA or BAPGM-kanamycin plates.
Bacteria were allowed to infect tick cells for approximately 24 h based on the observation of Mehock et al. (1998) who observed that 24 h was sufficient time for erythrocyte invasion by B. henselae. Twenty-four hours post-infection, two separate washes were performed to remove non-adherent bacteria. Medium from each well was replaced with 1 ml fresh medium and plates were rocked back and forth by hand for several minutes at room temperature to dislodge loosely adherent or non-adherent bacteria. Medium was removed and cells were washed a second time in the same manner. Upon completion of the washes, 1 ml L-15B300 medium was added to all wells containing parent strains. Again, 40 μg/ml of kanamycin was added to those wells containing B. henselae isolate A-GFP and B. henselae isolate B-GFP. The 24 h period following initial washes was considered day 0 post-wash. From day 0 post-wash until the completion of each study (day 5 post-wash), the entire contents of the wells (cells and supernatant) from an individual plate were collected daily and stored at −80°C until further analysis to quantify total growth for intra- and extra-cellular bacteria. Six plates were used per study; each plate represented a single post-wash day.
The ability of B. henselae isolates to invade and replicate within the I. scapularis (ISE6) and R. sanguineus (RSE8) cell lines was also examined, but growth kinetic studies were not performed. Replication and invasion of the bacteria within the cells were assessed visually and by cytospin analysis of infected supernatant using light microscopy.
Bartonella henselae growth in conditioned media
To analyze whether B. henselae can grow without the presence of tick cells, conditioned L-15B300 medium from 1 day old seeded cells was collected and then filtered through a 5 μm filter syringe (Pall Corporation, East Hills, NY, USA) to remove cellular debris. One milliliter of conditioned medium was aliquoted into nine wells of a 24 well plate (five plates total). Each plate contained triplicate wells for non-infected medium and medium infected with B. henselae isolate A and B. henselae isolate B. Non-transformed B. henselae isolates were grown for 5 days on TSA plates, resuspended in 5 ml of fresh L-15B300 medium (1.5–2.9 × 108 CFU/ml) after which wells were inoculated with 50 μl from each of the bacterial resuspensions into designated triplicate wells. Contents from all wells were collected daily for 5 days (day 1–5 post-infection) and frozen at −80°C until further analysis.
DNA extraction of supernatant from each well and qPCR
Total DNA was extracted from each B. henselae growth study sample (well) using a Qiagen BioRobot M48 workstation (Qiagen, Valencia, CA, USA). Samples were thawed at room temperature, vortexed, and 200 μl of inoculum was used for DNA extraction. For the BioRobot M48 workstation, a MagAttract DNA Blood Mini M48 kit was utilized and template DNA was resuspended in 100 μl of RNase-free water. DNA was extracted from each well only once.
A quantitative real-time PCR (qPCR) specific for the genus Bartonella as described by Duncan et al. (2008) was used to amplify a 120 bp fragment of the Bartonella 16S–23S intergenic spacer region (ITS). Briefly, 50 μl reaction volumes contained 19 μl of water, 25 μl of 2× SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA), 15 pmol of each primer (321 s: 5′ AGA TGA ATG ATC CCA AGC CTT CTG G 3′ and 425as: 5′ GAT AAA CCG GAA AAC CTT CCC 3′), and 5 μl of template DNA. Cycling conditions consisted of 95°C for 5 min, 45 cycles of 95°C for 30 s, 56°C for 30 s, 72°C for 30 s, and a final extension of 72°C for 5 min. To quantify the approximate number of organisms present per PCR reaction, ten fold serial dilutions of a known concentration of the 16S–23S ITS insert plasmid (109–0.1 plasmid copies) was resuspended in molecular grade water. Each plasmid dilution and each well supernatant extract was run in triplicate qPCR reactions.
Electron microscopy of Bartonella henselae
Twenty-four hours prior to infection with B. henselae isolates A and B, approximately 1.92 × 106 tick cells/flask were passaged into sealed 25 cm2 flasks. Tick cells were inoculated with 500 μl of the bacterial resuspension containing approximately 8.0 × 105–3.0 × 107 CFU/ml. Supernatant, not including attached cells, was collected at 24 h intervals up to day 4 post-infection and centrifuged to pellet cells and associated bacteria at 3,500 rpms for 5 min using a Marathon 16 km benchtop centrifuge (Fisher Scientific, Pittsburgh, PA, USA). Pellets were fixed with McDowell and Trump’s solution (4% formaldehyde and 1% glutaraldehyde in phosphate buffer) and processed according to standard techniques for transmission electron microscopy (Dykstra 1985). Non-infected cells passaged the same day were used as negative controls.
Infection of tick cells with different Bartonella species assessed by light microscopy
To assess the ability of different Bartonella species to infect AAE12 tick cells, Diff-Quik (Dade Behring, Aguando, PR, USA) staining was used to visualize bacteria within infected cells. Twenty-four hours prior to infection, 1.8 × 106–2.1 × 106 cells/flask were passaged into sealed 25 cm2 flasks. Isolates of B. bovis, Candidatus B. durdenii, B. elizabethae, Candidatus B. monaxi, B. vinsonii subsp. berkhoffii genotypes I–III, and Candidatus B. volans were used to determine whether a spectrum of host-adapted Bartonella spp. could invade AAE12 cells. Tick cells were inoculated with 500 μl of each bacterial resuspension containing approximately 1.3 × 108–9 CFU/ml on day of infection. On days 1–4 post-infection, 200 μl of supernatant, not including attached cells, was collected from each infected flask and bacterial cultures were analyzed using light microscopy study. Non-infected cell flasks, passaged the same day, were used as negative controls.
PCR amplification of bacterial cultures used for cytospin infection studies
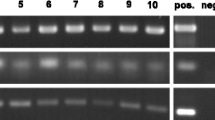
Screening of Bartonella species used for the cytospin studies was performed using conventional PCR from individual colonies grown on agar plates on the day of inoculation. Conventional PCR targeting the Bartonella species 16S–23S intergenic spacer region was performed as described in Billeter et al. (2008b). Each PCR reaction contained 14.25 μl of nuclease free water, 10× PCR buffer, 3 mM MgCl2, 15 pmol of each primer (325 s: 5′ CTT CAG ATG ATG ATC CCA AGC CTT TTG GCG 3′ and 1100as: 5′ GAA CCG ACG ACC CCC TGC TTG CAA AGC 3′), 0.2 mM dNTPs, and 1.25 U of HotStar Taq polymerase (Qiagen). Colonies were removed using a sterile pipet tip and resuspended in the reaction buffer. PCR conditions consisted of an initial cycle at 94°C for 15 min followed by 55 cycles of 94°C for 30 s, 66°C for 1 min, 72°C for 1 min, and a final cycle of 72°C for 5 min. PCR products were separated using 2% agarose gel containing ethidium bromide and visualized under UV light. The positive control consisted of B. henselae Houston-1 in AAE12 tick cell DNA and water as a negative control.
Statistical analysis
Bartonella DNA quantification was performed by comparing the threshold cycle (C t value) of each qPCR reaction with the C t value obtained from serial dilutions of quantified plasmid DNA. Since each whole Bartonella genome has two copies of the ITS region, quantifications were calculated by bacteria (genome equivalents) per qPCR reaction. Averages of three organism quantifications per replicate (well) were used for statistical analysis. For parametric analysis, data was transformed by logarithm with base ten. Data normality and homogeneity of variance were tested by Kolmogorov–Smirnov and Levene tests, respectively. Statistical differences between days of culture were assessed for parametric data using the one-way repeated measures ANOVA. Bonferroni t-test was used for multiple comparisons, in which days 1–5 post-wash were compared versus day 0 post-wash (control). The level of significance was set at P ≤ 0.05. Statistical analysis was performed with the aid of a statistical software package (SigmaStat for Windows, v.3.5.1, Chicago, IL).
Results
Growth of Bartonella henselae in tick cells and conditioned media
To assess the ability of B. henselae isolates A and B to grow within AAE12 tick cells, bacteria from frozen stocks were thawed and allowed to grow on agar plates for 4–7 days. Twenty-four hours prior to infection, tick cells were passaged into 15 wells of a 24 well plate. Triplicate wells were utilized for the culture negative “non-infected” controls and for each B. henselae isolate used to infect tick cells. Twenty-four hours post-infection, medium was removed and all cells were washed to remove non-adherent bacteria (Fig. 1). Cells and supernatant were collected daily until day 5 post-wash and were examined by quantitative qPCR to approximate the number of bacteria present per reaction. Figure 2 (1 and 2) represent the log (10) transformed growth data over the 5 day collection period. The parent isolates grew more efficiently than the GFP expressing isolates with a significant increase in growth for both parent strains on either day 2 (Fig. 2 (1), study 1 P = 0.014) or 3 (Fig. 2 (2), study 2 P = 0.005) post-wash. Differences in growth remained significant, as compared to the day 0 control, for the remainder of each study. There was no significant difference in growth, however, on day 0 post-wash and the remaining collection days of study 1 for either of the GFP transformed B. henselae isolates. There was a decrease in growth for B. henselae isolate A-GFP (Fig. 2 (2), study 2) between day 0 and day 1 post-wash (P = 0.046), though, no other differences could be found between later collection days.
Day 0 post-wash of B. henselae isolate A—GFP in Amblyomma americanum cell line, AAE12. Washes were performed to remove non-adherent bacteria 24 h post-infection. a Day 0 post-wash, or 1 day post-infection, demonstrating that GFP expressing B. henselae isolate A are cell associated or internalized. b Phase contrast of same field of B. henselae isolate A—GFP infected cells. c Fluorescent image of non-infected AAE12 cells. d Phase contrast of same field of non-infected cells. All images are at a magnification of 200×
Growth of Bartonella henselae in Amblyomma americanum cell line, AAE12. Growth of non-transformed B. henselae isolates A and B, and GFP expressing isolates, grown in AAE12 cells. AAE12 cells were infected 24 h prior to washes to remove non-adhered or loosely adherent bacteria. Cells and supernatant were collected daily from days 0–5 from three infected wells for each experimental group and analyzed by qPCR to determine number of organisms present per reaction. Different plates used for each collection day. Data represents the log (10) transformed mean from the three infected wells. ¤ difference in growth from day 0 post-wash for B. henselae isolate A and B (study 1 P = 0.014; study 2 P = 0.005, Bonferroni t-test), * difference in growth from day 0 post-wash for B. henselae isolate A and B—GFP (P = 0.046, Bonferroni t-test). Duplicate studies were performed: (1) study 1 and (2) study 2
After several days of infection, the non-transformed, or non-GFP expressing, B. henselae isolates were highly cytopathic to the tick cells; cell death began to occur within 2–3 days post-infection. By the end of both studies, most AAE12 cells were either lysed or heavily vacuolated, representative of a cell heavily infected with B. henselae. By day 3 post-infection, an overwhelming load of bacteria was present in cells and within the media. A similar tick cell cytopathic effect was not associated with B. henselae isolate A-GFP and B. henselae isolate B-GFP potentially because the GFP expressing bacteria did not replicate or did not replicate to the same level as the parent strains.
To determine if the non-transformed bacterial isolates could grow in conditioned media, triplicate wells containing medium from 1 day old cell cultures were infected with B. henselae isolate A and B. henselae isolate B. There was a significant increase in growth over the 5 days study period for both isolates (Fig. 3). Using the Bonferroni t-test, there was increased growth between day 1 (control) and day 5 post-infection for B. henselae isolate B (P = 0.016) and between days 1 and 2 and day 1 and 5 post-infection for B. henselae isolate A (P = 0.008 and P = 0.002, respectively).
Growth of non-transformed B. henselae in conditioned media. Growth of non-transformed B. henselae isolates grown in 1 day old seeded conditioned media. Media from infected and non-infected wells (3 wells/experimental group) was collected days 1–5 post-inoculation and analyzed by qPCR to determine the number of organisms present per reaction. Data represents the log (10) transformed mean from three infected wells. * Difference in growth from day 0 post-wash for B. henselae isolate A (P = 0.008, Bonferroni t-test); ¤ difference in growth from day 0 post-wash for B. henselae isolate B (P = 0.016, Bonferroni t-test)
Both B. henselae isolates were also able to invade and replicate within the ISE6 and RSE8 cell lines (data not shown). Both isolates were cytopathic to the host cells several days following infection. Furthermore, we have been able to subculture B. henselae through nine passages in the AAE12 cell line and 3–4 passages in the other tick cell lines (further passage attempts have not been made) over several months time using previously infected tick cells as our inoculum (data not shown).
Electron microscopic analysis
Infected tick cells harbored pleomorphic rod shaped bacteria, appearing to range in size from 0.8–1.2 μm in length to 0.3–0.6 μm in width, surrounded by a thin outer membrane (Fig. 4). There were a few morphological differences in the localization of intracellular bacteria between the two different B. henselae isolates. As seen in Fig. 4b, B. henselae isolate A bacteria were unevenly distributed throughout the cell and bacteria were not as tightly compacted within the tick cell as when the tick cells were infected with B. henselae isolate B (Fig. 4c). Furthermore, B. henselae isolate A bacteria appeared either loosely dispersed throughout the cytosol of the cell or more tightly clustered in morulae-like vacuoles (Fig. 4d). Bacteria of B. henselae isolate B were more evenly distributed throughout the cell and bacteria were either present in clusters throughout the cytoplasm or in morulae-like inclusions (Fig. 4e).
Electron micrographs of B. henselae isolate A and isolate B in AAE12 cells. Day 4 post-infection. a Non-infected cells. b B. henselae isolate A. c B. henselae isolate B. d B. henselae isolate A bacteria in membrane bound inclusion [(*) representative of these inclusion bodies)] e B. henselae isolate B bacteria present within the cytoplasm of the tick cell. N is representative of the nucleus of the cell. Thin arrow indicates mitochondria of the tick cell and the thick arrow is representative of bacteria present within the cell
Light microscopic analysis
To demonstrate internalization of Bartonella species within the AAE12 tick cells, cytospins from infected cultures, stained with Diff-Quik, were visualized using light microscopy. Dark blue inclusions present within the cytosol of infected cells were characteristic for internalized bacteria. The rod-shaped organisms present within the supernatant represented extra-cellular Bartonella (Fig. 5). Cytospin analysis demonstrated the ability of all examined Bartonella species to infect AAE12 tick cells, however, cytopathic effect on the host cells varied between the isolates. The Bartonella bovis isolate produced inclusion bodies within the cytoplasm of the tick cells; however, this isolate did not appear to inhibit cell survival. As illustrated in Fig. 5, the foamy appearance of the B. vinsonii subsp. berkhoffii genotype III infected cells was representative of heavily infected tick cells and was characteristic of infection by all Bartonella species examined, with the exception of B. bovis. Based upon the size of the PCR amplicon produced, the species of Bartonella used for each infection study was confirmed (data not shown).
Diff-Quik stain of cytospin slides of Bartonella-infected AAE12 cells. a B. bovis infected cells, day 4 post-infection. b B. vinsonii subsp. berkhoffii genotype III infected cells, day 3 post-infection. c Non-infected cells, day 5 post-passage. N is representative of the nucleus of the cell. Thin arrow indicates B. bovis inclusion bodies in cytosol of tick cells, thick arrow representative of bacteria in supernatant, (*) representative of highly infected cells. Note the foamy-like appearance of the heavily infected cells
Discussion
The A. americanum cell line, AAE12, was utilized to establish the ability of various Bartonella species to infect and replicate within a tick cell line. Using qPCR analysis, the growth of B. henselae within tick cells and within conditioned media could be determined over time. The B. henselae parent strains appeared to follow the expected bacterial growth curve (lag, log, and stationary phases) in the presence of tick cells, though this same growth trend was not evident within the conditioned media. Because different plates were utilized for each day of collection, it is possible that the decrease in detectable B. henselae isolate B between days 0 and 1 post-wash, for example, could be explained by between-plate variation in bacterial replication rate (Fig. 2 (1)). Utilization of 24 well plates allowed experiments to be performed on a more manageable scale than would have been possible if experiments had been performed using the 25 cm2 flasks. As illustrated in Fig. 3, there was also variability in detectable bacterial numbers for the parent strains of B. henselae grown in conditioned media. Interestingly, B. henselae isolate A and B did not appear to grow within non-conditioned media during preliminary studies when measured by qPCR analysis suggesting that while Bartonella are not intracellular obligate organisms, a biological component either secreted or associated with tick cells may be necessary for growth of B. henselae. The survivability of the bacteria in non-conditioned media over several days, however, was not simultaneously measured during these preliminary studies.
Both B. henselae–GFP strains grew very little, if at all, during the course of the studies. Reduced growth of other GFP expressing bacteria, compared to parent strains, have been observed in prior studies (Rang et al. 2003; Oscar et al. 2006). Oscar et al. (2006) observed a 40–50% reduction in growth between a GFP expressing Escherichia coli K-12 and the parent strain at three different temperatures. The GFP plasmid used to infect E. coli K-12 is a high copy plasmid that replicates extrachromosomally, and is similar to the GFP plasmid used in this study. The authors proposed that reduced growth of E. coli may not be related to the expression of the GFP protein, but instead due to bacterial transformation with the plasmid resulting in slower cellular division. Rang et al. (2003) suggested, however, that other factors not currently known should be considered. Because the detectable numbers of bacteria for both B. henselae isolates expressing GFP remained relatively stable, it appears the GFP constructs will be valuable for use in future studies.
By examining thin sections of infected tick cell culture by electron microscopy, we observed very few morphological differences in the localization of intracellular bacteria for two different isolates of B. henselae. Both B. henselae isolates A and B often appeared to form morulae-like inclusion bodies, collections of bacteria present within a clearly defined vacuole, which have previously only been identified within tick cells infected with Ehrlichia/Anaplasma species and Midichloria mitochondrii, a recently characterized bacterial endosymbiont of several tick species (Popov et al. 1998; Sassera et al. 2006; Epis et al. 2008). To our knowledge, this is the first description of morulae-like inclusions within a host cell that were induced by infection with a Bartonella species. Intracellular bacteria of B. henselae isolate B were often found tightly clustered within the cytoplasm or in the morulae-like inclusions, while bacteria of B. henselae isolate A were more loosely dispersed throughout the tick cell. Reasons for the few morphologic differences between the two B. henselae isolates within the tick cells are unclear, but may be due to currently unidentified genetic differences that influence the pattern of intracellular growth. Furthermore, tick cell lines are composed of mixed populations of cell types (Bell-Sakyi et al. 2007) which may explain the apparent morphological differences between the two B. henselae isolates as the cells observed in Fig. 4b, c are clearly different from one another. Recently, Cotté et al. (2008) demonstrated the potential transmission of B. henselae Houston-1 by I. ricinus ticks via feeding through an artificial membrane. Future research should address morphological comparisons between Bartonella henselae strains isolated from mammals and strains isolated from ticks when grown within tick cells.
Seven different Bartonella isolates and three Candidatus Bartonella species, maintained by different reservoir hosts and potentially transmitted by arthropod vectors other than ticks, were also able to infect and replicate within the AAE12 cell line as examined by Diff-Quik staining of infected culture. Depending upon the Bartonella species used, we observed different cytopathic effects within the AAE12 cells. B. bovis, thought to be transmitted by biting flies (Chung et al. 2004), produced inclusions within the cytosol and appeared to have minimal effect on host cell survival. With the exception of B. bovis, all Bartonella species induced vacuolization of tick cells eventually leading to individual cell lysis. Cell lysis was invariably associated with a heavy bacterial load within the cells.
Ohters have found differences in cytopathic effect to tick cells depending upon the infectious agent studied. Recently, using Diff-Quik staining, Pornwiroon et al. (2006) reported similar vacuolization in ISE6 cells infected with R. felis, bacteria transmitted by the cat flea, C. felis. In contrast to most Bartonella spp. examined in this study, R. felis did not induce cell lysis, though cells did contain a heavy burden of rickettisae. By decreasing bacterial inoculum size in preliminary studies, we observed an increase in the length of cell survival, however, infection by most Bartonella species did eventually lead to cell death. It remains unclear at this point whether Bartonella species utilized in this study actually invade the cells, or whether the bacteria were actively taken up by the cells themselves. It has been shown previously that some tick cell lines are naturally phagocytic (Mattila et al. 2007; Kurtti and Keyhani 2008). To determine whether Bartonella species can invade tick cells independently, further studies should be performed to compare the number of intracellular organisms present within tick cells after 24 h using live bacteria and dead, morphologically intact Bartonella. If Bartonella species are actively invading the tick cells, there should be an increase in live, intracellular bacteria over the 24 h period.
Isolation and propagation of Bartonella species has often proved difficult. Currently, a cell-free insect based medium, Bartonella alpha-Proteobacteria growth medium (BAPGM), is utilized within our laboratory for the isolation and growth of Bartonella species (Maggi et al. 2005). Use of insect cell culture-based media for the growth of Bartonella spp. was further validated by Riess et al. (2008). Direct inoculation of infected blood onto agar plates, use of shell vials, other liquid medium, and mammalian cell lines have also been used with variable success to isolate Bartonella species (Welch et al. 1992; Houpikian and Raoult 2002; Eremeeva et al. 2007; Kosoy et al. 2008). This is the first report, to our knowledge, detailing the infection and growth of Bartonella species within an arthropod cell line. Based upon the results of this study, the use of tick cell lines may further enhance the isolation and propagation of Bartonella species from clinical samples and may also facilitate studies that will increase our knowledge regarding Bartonella behavior within the arthropod vector. We are currently in the process of comparing the protein expression of B. henselae grown on plates to that of B. henselae grown within tick cells in order to identify potential differences in virulence factors. In 2007, Werner et al. (2007) performed experimental transmission studies using felines to determine whether B. henselae genotype I loses its virulence capabilities after 20–40 plate passages in vitro. Interestingly, there did not appear to be a difference in the induction time of bacteremia and no differences in the humoral immune responses between the cats examined. Tick-cell derived Bartonella antigens may prove useful for the development of more sensitive diagnostic reagents as current antibody tests appear to be relatively problematic and may assist in the development of an effective vaccine to prevent the further spread of disease caused by these organisms.
References
Bell-Sakyi L, Zweygarth E, Blouin EF, Gould EA, Jongejan F (2007) Tick cell lines: tools for tick and tick-borne disease research. Trends Parasitol 23:450–457. doi:10.1016/j.pt.2007.07.009
Bermond D, Boulouis H-J, Heller R, Van Laere G, Monteil H, Chomel BB, Sander A, Dehio C, Piémont Y (2002) Bartonella bovis Bermond et al. sp. nov. and Bartonella capreoli sp. nov., isolated from European ruminants. Int J Syst Evol Microbiol 52:382–390
Billeter SA, Levy MG, Chomel BB, Breitschwerdt EB (2008a) Vector transmission of Bartonella species with emphasis on the potential for tick transmission. Med Vet Entomol 22:1–15. doi:10.1111/j.1365-2915.2008.00713.x
Billeter SA, Miller MK, Breitschwerdt EB, Levy MG (2008b) Detection of two Bartonella tamiae-like sequences in Amblyomma americanum (Acari: Ixodidae) using 16S–23S intergenic spacer region-specific primers. J Med Entomol 45:176–179. doi:10.1603/0022-2585(2008)45[176:DOTBTS]2.0.CO;2
Blouin EF, Kocan KM (1998) Morphology and development of Anaplasma marginale (Rickettsiales: Anaplasmataceae) in cultured Ixodes scapularis (Acari: Ixodidae) cells. J Med Entomol 35:788–797
Chang CC, Chomel BB, Kasten RW, Romano V, Tietze N (2001) Molecular evidence of Bartonella spp. in questing adult Ixodes pacificus ticks in California. J Clin Microbiol 39:1221–1226. doi:10.1128/JCM.39.4.1221-1226.2001
Chomel BB, Kasten RW, Floyd-Hawkins K, Chi B, Yamamoto K, Roberts-Wilson J, Gurfield AN, Abbott RC, Pedersen NC, Koehler JE (1996) Experimental transmission of Bartonella henselae by the cat flea. J Clin Microbiol 34:1952–1956
Chung CY, Kasten RW, Paff SM, Van Horn BA, Vayssier-Taussat M, Boulouis H-J, Chomel BB (2004) Bartonella spp. DNA associated with biting flies from California. Emerg Infect Dis 10:1311–1313
Cotté S, Bonnet S, Le Rhun D, Le Naour E, Chauvin A, Boulouis HJ, Lecuelle B, Lilin T, Vayssier-Taussat M (2008) Transmission of Bartonella henselae by Ixodes ricinus. Emerg Infect Dis 14:1074–1080. doi:10.3201/eid1407.071110
de la Fuente J, Kocan KM, Garcia-Garcia JC, Blouin EF, Claypool PL, Saliki JT (2002) Vaccination of cattle with Anaplasma marginale derived from tick cell culture and bovine erythrocytes followed by challenge-exposure with infected ticks. Vet Microbiol 89:239–251. doi:10.1016/S0378-1135(02)00206-7
Duncan AW, Marr HS, Birkenheuer AJ, Maggi RG, Williams LE, Correa MT, Breitschwerdt EB (2008) Bartonella DNA in the blood and lymph nodes of golden retrievers with lymphoma and in healthy controls. J Vet Intern Med 22:89–95. doi:10.1111/j.1939-1676.2007.0018.x
Dykstra MJ (1985) A manual of applied techniques for biological electron microscopy. Plenum Publishing Corp, New York
Ellis BA, Regnery RL, Beati L, Bacellar F, Rood M, Glass GG, Marston E, Ksiazek TG, Jones D, Childs JE (1999) Rats of the genus Rattus are reservoir hosts for pathogenic Bartonella species: an Old World origin for a New World disease? J Infect Dis 180:220–224. doi:10.1086/314824
Epis S, Sassera D, Beninati T, Lo N, Beati L, Piesman J, Rinaldi L, McCoy KD, Torina A, Sacchi L, Clementi E, Genchi M, Magnino S, Bandi C (2008) Midichloria mitochondrii is widespread in hard ticks (Ixodidae) and resides in the mitochondria of phylogenetically diverse speceies. Parasitology 135:485–494
Eremeeva ME, Gerns HL, Lydy SL, Goo JS, Ryan ET, Matthew SS, Ferraro MJ, Holden JM, Nicholson WL, Dasch GA, Koehler JE (2007) Bacteremia, fever, and splenomegaly caused by a newly recognized Bartonella species. N Engl J Med 356:2381–2387. doi:10.1056/NEJMoa065987
Fournier PE, Minnick M, Raoult D (1999) Transformation of Bartonella quintana to green fluorescent protein expression by electroporation. In: Raoult D, Brouqui P (eds) Rickettsiae and rickettsial diseases at the turn of the third millenium. Elsevier, Marseille, France, pp 38–42
Houpikian P, Raoult D (2002) Diagnostic methods current best practices and guidelines for identification of difficult-to-culture pathogens in infective endocarditis. Infect Dis Clin North Am 16:377–392. doi:10.1016/S0891-5520(01)00010-1
Kocan KM, Halbur T, Blouin EF, Onet V, de la Fuente J, Garcia-Garcia JC, Saliki JT (2001) Immunization of cattle with Anaplasma marginale derived from tick cell culture. Vet Parasitol 102:151–161. doi:10.1016/S0304-4017(01)00519-2
Kordick DL, Swaminathan B, Greene CE, Wilson KH, Whitney AM, O’Connor S, Hollis DG, Matar GM, Steigerwalt AG, Malcolm GB, Hayes PS, Hadfield TL, Breitschwerdt EB, Brenner DJ (1996) Bartonella vinsonii subsp. berkhoffii subsp. nov., isolated from dogs; Bartonella vinsonii subsp. vinsonii; and emended description of Bartonella vinsonii. Int J Syst Bacteriol 46:704–709
Kosoy M, Morway C, Sheff KW, Bai Y, Colburn J, Chalcraft L, Dowell SF, Peruski LF, Maloney SA, Baggett H, Sutthirattana S, Sidhirat A, Maruyama S, Kabeya H, Chomel BB, Kasten R, Popov V, Robinson J, Kruglov A, Petersen LR (2008) Bartonella tamiae sp. nov., a newly recognized pathogen isolated from three human patients from Thailand. J Clin Microbiol 46:772–775. doi:10.1128/JCM.02120-07
Kurtti TJ, Keyhani NO (2008) Intracellular infection of tick cell lines by the entomopathogenic fungus Metarhizium anisopliae. Microbiology 154:1700–1709
Kurtti TJ, Munderloh UG, Samish M (1982) Effect of medium supplements on tick cells in culture. J Parasitol 68:930–935. doi:10.2307/3281008
Lucey D, Dolan MJ, Moss CW, Garcia M, Hollis DG, Wegner S, Morgan G, Almeida R, Leong D, Geisen KS, Welch DF, Slater DN (1992) Relapsing illness due to Rochalimae henselae in immuncompetent hosts: implication for therapy and new epidemiological associations. Clin Infect Dis 14:683–688
Maggi RG, Duncan AW, Breitschwerdt EB (2005) Novel chemically modified liquid medium that will support the growth of seven Bartonella species. J Clin Microbiol 43:2651–2655. doi:10.1128/JCM.43.6.2651-2655.2005
Maggi RG, Chomel B, Hegarty BC, Henn J, Breitschwerdt EB (2006) A Bartonella vinsonii berkhoffii typing scheme based upon 16S–23S ITS and Pap31 sequences from dog, coyote, gray fox, and human isolates. Mol Cell Probes 20:128–134. doi:10.1016/j.mcp.2005.11.002
Marié JL, Fournier PE, Rolain JM, Briolant S, Davoust B, Raoult D (2006) Molecular detection of Bartonella quintana, B. elizabethae, B. koehlerae, B. doshiae, B. taylorii, and Rickettsia felis in rodent fleas collected in Kabul, Afghanistan. Am J Trop Med Hyg 74:436–439
Mattila JT, Munderloh UG, Kurtti TJ (2007) Phagocytosis of the Lyme disease spirochete, Borrelia burgdorferi, by cells from the ticks, Ixodes scapularis and Dermacentor andersoni, infected with an endosymbiont, Rickettsia peacockii. J Insect Sci 7:58
Mehock JR, Greene CE, Gherardin FC, Hahn TW, Krause DC (1998) Bartonella henselae invasion of feline erythrocytes in vitro. Infect Immun 66:3462–3466
Munderloh UG, Blouin EF, Kocan KM, Ge NL, Edwards WL, Kurttti TJ (1996) Establishment of the tick (Acari: Ixodidae)-borne cattle pathogen Anaplasma marginale (Rickettsiales: Anaplasmataceae) in tick cell culture. J Med Entomol 33:656–664
Munderloh UG, Jauron SD, Fingerle V, Leitritz L, Hayes SF, Hautman JM, Nelson CM, Huberty BW, Kurtti TJ, Ahlstrand GG, Greig B, Mellencamp MA, Goodman JL (1999) Invasion and intracellular development of the human granulocytic ehrlichiosis agent in tick cell culture. J Clin Microbiol 37:2518–2524
Oscar TP, Dulal K, Boucaud D (2006) Transformation of Escherichia coli K-12 with a high-copy plasmid encoding the green fluorescent protein reduces growth: implications for predictive microbiology. J Food Prot 69:276–281
Parola P, Shpynov S, Montoya M, Lopez M, Houpikian P, Zeaiter Z, Guerra H, Raoult D (2002) First molecular evidence of new Bartonella spp. in fleas and a tick from Peru. Am J Trop Med Hyg 67:135–136
Popov VL, Han VC, Chen SM, Dumler JS, Feng HM, Andreadis TG, Tesh RB, Walker DH (1998) Ultrastructural differentiation of the genogroups in the genus Ehrlichia. J Med Microbiol 47:235–251
Pornwiroon W, Pourciau SS, Foil LD, Macaluso KR (2006) Rickettsia felis from cat fleas: isolation and culture in tick-derived cell line. Appl Environ Microbiol 72:5589–5595. doi:10.1128/AEM.00532-06
Rang C, Galen JE, Kaper JB, Chao L (2003) Fitness cost of the green fluorescent protein in gastrointestinal bacteria. Can J Microbiol 49:531–537. doi:10.1139/w03-072
Reeves WK, Nelder MP, Korecki JA (2005) Bartonella and Rickettsia in fleas and lice from mammals in South Carolina, USA. J Vector Ecol 30:310–315
Regnery R, Martin M, Olson J (1992) Naturally occurring “Rochalimaea henselae” infection in domestic cat. Lancet 340:557–558. doi:10.1016/0140-6736(92)91760-6
Riess T, Raddatz G, Linke D, Schäfer A, Kempf VA (2008) Analysis of a novel insect cell culture medium-based growth for Bartonella species. Appl Environ Microbiol 74:5224–5227. doi:10.1128/AEM.00621-08
Saliki JT, Blouin EF, Rodgers SJ, Kocan KM (1998) Use of tick cell culture-derived Anaplasma marginale antigen in a competititive ELISA for serodiagnosis of anaplasmosis. Ann N Y Acad Sci 849:273–281. doi:10.1111/j.1749-6632.1998.tb11059.x
Sassera D, Beninati T, Bandi C, Bouman EAP, Sacchi L, Fabbi M, Nathan Lo (2006) ‘Candidatus Midichloria midochondrii’, an endosymbiont of the tick Ixodes ricinus with a unique intramitochondrial lifestyle. Int J Syst Evol Microbiol 56:2535–2540. doi:10.1099/ijs.0.64386-0
Tate CM, Mead DG, Luttrell MP, Howerth EW, Dugan VG, Munderloh UG, Davidson WR (2005) Experimental infection of white-tailed deer with Anaplasma phagocytophilum, etiologic agent of human granulocytic anaplasmosis. J Clin Microbiol 43:3595–3601. doi:10.1128/JCM.43.8.3595-3601.2005
Varela AS, Luttrell MP, Howerth EW, Moore VA, Davidson WR, Stallknecht DE, Little SE (2004) First culture isolation of Borrelia lonestari, putative agent of southern tick-associated rash illness. J Clin Microbiol 42:1163–1169. doi:10.1128/JCM.42.3.1163-1169.2004
Welch DF, Pickett DA, Slater LN, Steigerwalt AG, Brenner DJ (1992) Rochalimaea henselae sp. nov., a cause of septicemia, bacillary angiomatosis, and parenchymal bacillary peliosis. J Clin Microbiol 30:275–280
Werner JA, Kasten RW, Feng S, Sykes JE, Hodzic E, Salemi MR, Barthold SW, Chomel BB (2007) Experimental infecton of domestic cats with passaged genotype I Bartonella henselae. Vet Microbiol 3–4:290–297
Acknowledgments
This study was supported in part by a grant from the North Carolina State University Center for Comparative Medicine and Translational Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Billeter, S.A., Diniz, P.P.V.P., Battisti, J.M. et al. Infection and replication of Bartonella species within a tick cell line. Exp Appl Acarol 49, 193–208 (2009). https://doi.org/10.1007/s10493-009-9255-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-009-9255-1