Abstract
The genus Aureobasidium, which is known as a wood staining mould, has been detected on oil treated woods in the specific stain formation called biofinish. This biofinish is used to develop a new protective, self-healing and decorative biotreatment for wood. In order to understand and control biofinish formation on oil treated wood, the occurrence of different Aureobasidium species on various wood surfaces was studied. Phenotypic variability within Aureobasidium strains presented limitations of morphological identification of Aureobasidium species. PCR amplification and Sanger sequencing of ITS and RPB2 were used to identify the culturable Aureobasidium species composition in mould stained wood surfaces with and without a biofinish. The analysed isolates showed that several Aureobasidium species were present and that Aureobasidium melanogenum was predominantly detected, regardless of the presence of a biofinish and the type of substrate. A. melanogenum was detected on wood samples exposed in the Netherlands, Cameroon, South Africa, Australia and Norway. ITS-specific PCR amplification, cloning and sequencing of DNA extracted from biofinish samples confirmed results of the culturing based method: A. melanogenum is predominant within the Aureobasidium population of biofinishes on pine sapwood treated with raw linseed oil and the outdoor placement in the Netherlands.
Similar content being viewed by others
Introduction
Aureobasidium are wood staining fungi, in particular on wood situated outdoors above the ground (Dickinson 1972; Dix and Webster 1995; Bardage 1998; Schmidt 2006; Gobakken and Westin 2008). The interest in Aureobasidium has recently increased, because of its role in the formation of biofinishes on wood (Sailer et al. 2010; van Nieuwenhuijzen et al. 2013; van Nieuwenhuijzen et al. 2015; Filippovych et al. 2015). The term biofinish was introduced for a uniform dark mould covering which emerged outdoors on oil treated wood (van Nieuwenhuijzen et al. 2015). Although the protection mechanism and durability of this biofinish is still under investigation, biofinished wood is considered to be an appealing biocide-free construction material that has the advantage of also having self-healing properties.
Until now it is unknown which Aureobasidium species participates in the biofinish formation and whether a biofinish is composed of more than a single species. Although Aureobasidium has been isolated from many organic and inorganic substrates and geographical locations (Zalar et al. 2008; Slepecky and Starmer 2009; Gaur et al. 2010), the ubiquity of the specific species is unknown. The geographical location, the combination of wood species and the oil treatment may all have an impact on the species composition of the Aureobasidium population in biofinishes. Species-specific behaviour, such as phenotype and physiology (Samson et al. 2010; Houbraken 2013), should be included in future research in order to understand and control dark mould growth on oil treated wood. Therefore insight in the species composition of the biofinish is highly relevant.
The ascomycete genus Aureobasidium is a member of the family Aureobasidiaceae within the class of the Dothideomycetes (Thambugala et al. 2014; Wijayawardene et al. 2014). Kabatiella is closely related to Aureobasidium based on morphology and phylogeny (Zalar et al. 2008; Bills et al. 2012; Crous et al. 2011; Peterson et al. 2013; Thambugala et al. 2014) and some of these Kabatiella species may belong to Aureobasidium (Peterson et al. 2013; Thambugala et al. 2014). In addition future studies may result in the transfer of the species Selenophoma mahoniae and Columnosphaeria (Discosphaerina) fagi into Aureobasidium (Yurlova et al. 1999; Peterson et al. 2013; Thambugala et al. 2014). A well-known Aureobasidium species is Aureobasidium pullulans (Zalar et al. 2008; Gostinčar et al. 2014). The total number of classified Aureobasidium species currently varies per database, for example 38 in MycoBank and 13 in GenBank (October 2015).
Before DNA sequencing was applied in fungal taxonomy, the species classification system was mainly based on physiologic and phenotypic characteristics. In the case of Aureobasidium, colony pigmentation was used as a species-specific phenotypic characteristic (Zalar et al. 2008; Peterson et al. 2013). Nowadays, also the genealogical concordance phylogenetic species recognition (GCPSR) concept is commonly applied for species delimitation (Taylor et al. 2000). For species delimitation according to GCPSR multigene phylogenies are required. Next to the large subunit and the internal transcribed spacer regions (incl. 5.8S rDNA) (ITS) more variable genes such as translation elongation factor 1α, β-tubulin and RNA polymerase II- second largest subunit (RPB2) have been applied or recommended for phylogenetic analysis of Aureobasidium species (Zalar et al. 2008; Manitchotpisit et al. 2009; Peterson et al. 2013; Gostinčar et al. 2014). A phylogeny, including all described genera and species within the Aureobasidiaceae, is not yet available.
The ITS locus is assigned as the primary barcode for fungal species (Schoch et al. 2012). A large number of ITS barcode sequences of Aureobasidium species is available in public databases, which makes this DNA region a suitable marker to identify Aureobasidium (Manitchotpisit et al. 2009). To date, no second fungal barcode has been determined for a reliable Aureobasidium identification on species level.
The aim of this study was to explore the Aureobasidium species composition of biofinishes on wood. Culturable Aureobasidium isolates, retrieved from substrates with and without biofinishes, were identified. The wood species, oil treatments and exposure sites were related to the culturable species composition. Also direct extraction of biofinish DNA, followed by ITS amplification, cloning and sequencing were used to determine the species compositions of biofinishes.
Materials and methods
Substrates and outdoor exposure
Nine sample sets were analysed in this study. Each set contained oil treated wood samples. Untreated pine sapwood and glass were also selected for several sample sets (Fig. 1; Table 1), representing oil-free organic and inorganic materials that are associated with Aureobasidium growth (Gorbushina and Palinska 1999; Schabereiter-Gurtner et al. 2001; van Nieuwenhuijzen et al. 2015). The amount of different substrates (e.g. wood species, oil type), the geographical location of the outdoor exposure and exposure time was specific for each sample set (Table 1).
The wood species tested were pine (Pinus sylvestris) sapwood, spruce (Picea abies) and ilomba (Pycnanthus angolensis). No specific sapwood or heartwood selection was made for the latter two species. The surfaces of the wood samples were planed. The samples measured 5 cm (longitudinal axis), × 2.5 cm × 1.5 cm, except the specimens of set 4 which measured 10 cm × 14 cm × 2 cm. Glass sheets (Fisher Scientific) measured 10 cm × 10 cm × 0.3 cm.
Three different vegetable oil types were used to impregnate the wood specimens: raw linseed oil (Vereenigde Oliefabrieken; iodine value 183 and 0.81 % free fatty acids), olive oil (two brands: in case of sample set 4 unfiltered olive oil of 100 % Carolea olive, Calabrië EV Bio 2013; for the other sample sets Carbonel, extra vierge, iodine value 82 and 0.34 % free fatty acids), and stand linseed oil (Vliegenthart, viscosity P45). To determine the average moisture content right before impregnation, additional untreated test pieces of wood were dried at 105 °C. The moisture content of these wood pieces was up to 12 %. The impregnation of the small specimens (5 cm × 2.5 cm × 1.5 cm) was carried out using a vacuum time of 30 min at −1 bar followed by 1 h pressure of 8 bar. A vacuum time of 1 h at −1 bar followed by 2 h pressure of 8 bar was used for the larger specimens of sample set 4.
The wood samples of set 1–3 were steamed twice with hot air for 20 min on two consecutive days (European Standard 1996; Fritsche and Laplace 1999). The glass sheets were cleaned with alcohol and autoclaved before outdoor exposure. No sterilisation method was applied to the wood samples of set 4–9.
Five sites, located in different countries (Table 1) were used for outdoor exposure. The samples from set 4 remained outdoors during the biomass removal. Specifications on outdoor exposure and handling procedures were described in van Nieuwenhuijzen et al. (2015).
Biofinish assessment
The samples of set 5 and 7–9 have previously been evaluated for biofinish formation in the study of van Nieuwenhuijzen et al. (2015). This method consisted of visual analysis of the stain coverage on the surface as well as in situ spectrophotometer measurements of the pigmentation. In short a biofinish was assigned when the stain coverage was above 90 % and the pigmentation, expressed by triplets as used in the sRGB colour space, met the following criteria: all the red (R), green (G) and blue (B) values were below 82 and the difference between two values of a single RGB triplet was below 20. In comparison the RGB triplet of ultimate black was [0,0,0] and ultimate white was [255,255,255]. The biofinish assessment was also applied on the samples of set 6. The presence of a biofinish on the wood samples of set 1–4 was determined according to the surface coverage part of the biofinish assessment. A full biofinish assessment of the samples of set 4 was performed three months after fungal isolation.
Collection of Aureobasidium isolates
Within a sample set, up to two specimens per substrate were used for isolation (Table 1). The swab sampling method as described in van Nieuwenhuijzen et al. (2015) was used to collect biomass. Biomass suspensions were plated on malt extract agar (MEA) supplemented with penicillin and streptomycin (P/S) and on dichloran 18 % glycerol agar (DG18). The formulation of the agar media were according to Samson et al. (2004) and the plates were incubated at 25 °C for 14 days. A selection of the colonies, that phenotypically resembled Aureobasidium, was transferred to new MEA plates. The phenotypic characteristics used to determine Aureobasidium colonies: fast growing, yeast like colonies with an irregular edge, either white/pale pink coloured colonies mostly with a black centre and/or sectors or black coloured colonies with a small white boundary; white aerial hyphae sometimes present. Isolates of the selected colonies were deposited in the working collection of the Department of Applied and Industrial Mycology (DTO) housed at the CBS-KNAW Fungal Biodiversity Centre.
Phenotypic diversity of Aureobasidium strain DTO 217-G5
A large phenotypic variation within the Aureobasidium colonies on agar plates was observed during isolation. The isolate DTO 217-G5 (= CBS 140241) was used to study the phenotypic variability of a single strain. It was selected as a representative of the black cultures obtained from oil treated wood in the initial stage of biofinish formation. At first biomass was obtained of the edge of a 7 days old colony on MEA and washed in ultrapure water twice before dilution in ultrapure water. This dilution was combined with 10 × Yeast Nitrogen Base (Difco Laboratories 1998) with no additional carbon source and transferred to a shake flask. Due to the limited amount of carbon, the strain was cultivated in a nutrient limited and therefore stressful environment. After 24 h of shaking at 175 rpm at 25 °C, a serial dilution was made of the cell suspension and plated on oatmeal agar. After 5 days of incubation at 25 °C, four phenotypically diverse colony forming units (CFU’s) were selected as parental colonies and inoculated on MEA P/S (first MEA P/S inoculation). After incubation each colony was transferred to a new MEA P/S plate in triplicate. These colonies on the new plates were again transferred to MEA P/S three times in succession. Phenotypically diverse areas were selected for the biomass transfers. Photos were made and ITS sequences generated (as described below) of the colonies of the first and last inoculation on MEA P/S.
DNA extraction, amplification and sequencing
Isolates were grown on MEA plates prior to DNA extraction. DNA was extracted using the Ultraclean Microbial DNA isolation kit (MoBio Laboratories, Carlsbad, CA, USA) according to the manufacturer’s instructions. The ITS and RPB2 fragments were amplified using the primer pairs V9G (de Hoog and Gerrits van den Ende 1998) & LS266 (Masclaux et al. 1995) and RPB-PenR1 & RPB-PenR2 (Manitchotpisit et al. 2009). The PCR reactions were performed according to van Nieuwenhuijzen et al. (2015). The RPB2-PCR program differed by a primer annealing at 54 °C for 60 s. The amplified DNA fragments were sequenced and assembled as described in Yilmaz et al. (2014). Generated sequences are deposited in GenBank. The accession numbers of the new outdoor isolates are included in Table 2.
Phylogenetic analysis and identification of isolates
Reference strains of species which were used to generate a benchmark for the molecular identification of the Aureobasidium isolates are listed in Table 3. The GenBank accession numbers of the sequences are included in the table, except for the sequences of the Aureobasidium thailandense strains generated by Peterson et al. (2013; TreeBASE SN4236). The ITS and RPB2 sequence data sets were aligned using the program Muscle within MEGA version 5 (Tamura et al. 2011). Maximum Likelihood (ML) analysis was performed using MEGA. The number of bootstrap replicates was set on 1000. Sydowia polyspora CBS 750.71 was selected as outgroup. The isolates were identified based on the clustering in the phylogenetic trees with the type and other representative strains. A bootstrap value of 70 % or more was considered as moderated support for the identification of clades.
PCR, cloning and sequencing of biofinish DNA
ITS-specific cloning libraries were made of biofinishes of two types of substrates of set 5 in triplicate: pine sapwood & raw linseed oil (library PRL.1–PRL.3) and pine sapwood & olive oil (library PO.1–PO.3). An area of 2.5 cm × 2.5 cm of the upper surface of a specimen was scratched with a scalpel and DNA was extracted of the obtained biomass. The DNA extraction method, ITS primers and PCR-program were identical to the method described above. The PCR master mixes with ITS primers were prepared with the GoTaq Long PCR Master Mix (Progema) according to the manufacturer’s instructions. The PCR products were purified with the QIAquick PCR purification kit. Following the manufacturer’s instructions, 45 ng of PCR products was ligated and cloned (pGEM®-T Easy Vector Systems) into an Escherichia coli plasmid library. After growing ITS containing competent cells on plate, colonies were aseptically transferred to 10 μl demineralised water. PCR reactions were performed in 25 μl reaction mixtures containing 3 μL aliquots with ITS DNA, 2.5 μl PCR buffer, 2 μl MgCl2 (25 mM), 11 μl demineralised sterile water, 5 μL dNTP (1 mM), 0.50 μl of each primer (10 µM) and 0.5 μl Taq polymerase (5 U/μL, Bioline). The ITS-PCR program, sequencing, and assembling were similar to the previously described method. Assembled ITS sequences were generated of 62–69 cloned colonies per wood sample library. The ITS sequences of the cloning libraries were screened against the non-redundant NCBI database, using the program BLASTN. Sequences resulting in hits with an identity of 97 % or more compared to Aureobasidium sequences of the database were used for phylogenetic analysis. Sequences were submitted to GenBank (Table 4).
Results
Biofinish assessment
All wood samples of sample set 3–9 showed dark discolorations, but a biofinish was only established on a few samples (Table 5). Biofinishes were detected on specific samples exposed for more than one year at the sample site in the Netherlands (sample set 4 and 5): pine sapwood samples treated with raw linseed oil or olive oil and spruce and ilomba samples treated with olive oil. Furthermore, biofinishes were detected on the pine sapwood sample treated with olive oil that was exposed in South Africa (sample set 7) and the pine sapwood sample treated with raw linseed oil that was exposed in Norway (sample set 9).
Collection of Aureobasidium isolates
The number of isolates used in this study varied per substrate of each set (Table 5). These isolates were obtained from CFU’s on agar plates after culturing biomass of the substrates. They represent a small number of all the CFU’s which phenotypically resembled Aureobasidium species. For example 7–10 isolates were studied per sample in set 5 (Table 5), while the total amount of the counted Aureobasidium CFU’s on MEA and DG18 was up to 9 × 103 per sampled surface (van Nieuwenhuijzen et al. publication in progress).
Phenotypic diversity of Aureobasidium strain DTO 217-G5
The macromorphology of various inoculations of DTO 217-G5 (= CBS 140241) were compared to study the limitations of a phenotypic classification method for Aureobasidium species. The ITS barcode of isolate DTO 217-G5 and all its inoculations were identical. Based on this data, the isolate was identified as Aureobasidium melanogenum (Supplementary Data Fig. 1). The studied colonies of DTO 217-G5 (Fig. 2) were considered to be pure single strains since they were obtained as CFU’s after plating a serial diluted yeast-like cell suspension. After the first transfer of four of the CFU’s, which had different phenotypic characteristics on OA, to MEA P/S plates all colonies showed dark pigmentation and aerial hyphae in the margin and a more or less equal colony diameter (at 6 days of incubation). The colony texture, degree of pigmentation and mycelial production varied. The colonies after another three consecutive times of transfer and incubation showed more variation in their macromorphology. Although almost all examined cultures showed dark pigmentation, the degree varied widely and was even absent in one culture. Also the colony surface area and appearance varied. Some colonies produced aerial hyphae at the margin and the degree of hyphal production varied between isolates. Furthermore, the slimy appearance of the colonies which is described as cultural characteristic of A. melanogenum (Zalar et al. 2008), was also absent in some cultures.
Identification of Aureobasidium isolates
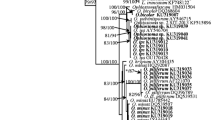
The majority of all 222 sequenced Aureobasidium isolates could be unambiguously identified (Fig. 3). Aureobasidium proteae and Columnosphaeria fagi resided in a clade with A. pullulans (Fig. 3) and are considered as synonyms of A. pullulans. The majority of the strains clustered together with the type of A. melanogenum (CBS 105.22T). Eleven strains had similar sequences as the type of A. melanogenum; however, these strains couldn’t be confidentially resolved in the A. melanogenum clade (bootstrap values below 7 0 %, Fig. 3). The sequence variation could be fully attributed to the RPB2 part of the concatenated sequences. These strains were therefore identified as Aureobasidium confer (cf.) melanogenum. Three clades with moderate bootstrap support (Fig. 3) did not contain any type or other reference strains and the isolates in these groups were tentatively named Aureobasidium sp. 1, sp. 2 and sp. 3. Sequences of strains named K. microsticta, K. harpospora, and K. zeae were excluded from the Aureobasidium phylogenetic overview. Kabatiella microsticta was represented by two strains that were placed in two far apart clades in the phylogenetic tree while none of these strains were classified as type strain. The latter two Kabatiella species were closer related to the outgroup than to the other Aureobasidium species.
Interestingly, 18 of the 222 Aureobasidium isolates had ambiguous nucleotide sites in their RPB2 sequences. Eleven of these isolates were identified as A. melanogenum, one as A. pullulans and six as Aureobasidium sp. 1. The bootstrap values were above 70 % (Supplementary Data Fig. 2).
Aureobasidium species composition on stained wood surfaces
Isolates from the biofinish containing wood samples revealed that all six biofinishes contained A. melanogenum (Fig. 4). Other detected species were A. leucospermi, A. namibiae and A. pullulans. The isolates consisted of 42 Aureobasidium colonies that were selected after culturing biomass from biofinish containing wood. 81 % of these isolates were identified as A. melanogenum.
Isolates from the 27 wood specimens, which contained visual mould staining but did not have a biofinish, showed that 80 % of these wood specimens contained A. melanogenum. In addition to this species, also A. cf. melanogenum, A. namibiae, A. pullulans, K. lini, and Aureobasidium sp. 1, sp. 2, and sp. 3 were detected on the mould stained wood samples without biofinish (Fig. 4). These isolates consisted of 110 Aureobasidium colonies that were selected after culturing biomass from the sample surfaces. 60 % of these isolates were identified as A. melanogenum.
Impact of different wood and (oil-) treatments on the species composition
A. melanogenum was detected on 21 of the 25 wood samples of sets 4 and 6–9 after exposure at the five different sites. A. melanogenum was (one of) the most detected species for each substrate. Per substrate 3–6 other species were found. The species were identified as A. leucospermi, A. namibiae, A. pullulans, A. cf. melanogenum, Aureobasidium sp. 1, Aureobasidium sp. 2, Aureobasidium sp. 3 or K. lini. These species were in most cases detected on one to two samples per substrate.
Impact of exposure sites on the species occurrence
A. melanogenum was detected in the sample sets exposed outdoors in the Netherlands, Cameroon, South Africa, Australia and Norway (Fig. 5). Other Aureobasidium species were detected as well, but were not obtained from all locations (Fig. 5). This outcome could be influenced by the limited number of isolates analysed per location. For example, 13–25 isolates were obtained from samples exposed at sites outside the Netherlands (Table 5).
Samples exposed in Australia and South Africa contained the highest Aureobasidium species diversity (Fig. 5). In the Netherlands, only A. melanogenum and A. pullulans isolates were detected, despite the relatively high number of substrate types (9) and identified isolates (149). This indicates that the detectable species diversity of outdoor placed substrates is influenced by the location of the exposure site.
Aureobasidium species composition on oil treated wood in time
The isolates from the pine sapwood samples treated with raw linseed oil (sample sets 1–4) showed that the number of colonies identified as A. pullulans decreased over time and the number of colonies identified as A. melanogenum increased over time (Fig. 6). At 5 and 12 months of outdoor exposure of the samples, when mould staining on the wood surface was present, the majority of the corresponding analysed colonies were identified as A. melanogenum. This in contrast to the results of the analysed colonies isolated from the reference material glass and the pine sapwood samples that had a shorter exposure time. More than 80 % of a colony set retrieved from glass was identified as A. pullulans regardless the exposition time.
PCR, cloning and sequencing of biofinish DNA
In order to analyse the Aureobasidium species composition of biofinishes on wood without a cultivation step, cloning libraries were generated of the DNA of six biofinishes. Each of the six cloning libraries contained clones with ITS DNA that belonged to several genera. In all libraries at least one sequence was identified as Aureobasidium by BLASTN on the NCBI database. Most of the sequences obtained from clones with Aureobasidium DNA clustered together in the phylogenetic trees with either the two A. melanogenum or the A. pullulans reference strains (bootstrap values above 63 %). A few Aureobasidium sequences (PRL.1.31, PRL.1.69, PRL3.16, PRL.3.19, PRL.3.70 and PO1.75) could not be identified on species level (Table 6), because they did not cluster with any of the known species. Further investigation revealed that these sequences contained parts of more than 100 nucleotides that differed largely from the reference strains.
The cloning libraries of the biofinishes on the samples treated with raw linseed oil had more than 50 % of all 62–69 clones per library identified as Aureobasidium. The predominant species of these cloning libraries was A. melanogenum (Table 6). The predominant species within the Aureobasidium DNA of biofinishes obtained from pine sapwood samples treated with olive oil remains unclear. Firstly the amount of clones identified as Aureobasidium per library was much lower, varying from 1 to 8 clones, and secondly one library showed the number of clones identified as A. pullulans to be equal to the ones identified as A. melanogenum (Table 6).
Discussion
Identification of Aureobasidium species
Morphology
Different phenotypic characteristics are described per Aureobasidium species (Zalar et al. 2008; Samson et al. 2010). The deviation of the general macromorphological characteristics of a strain within an Aureobasidium species can be explained by degeneration (Zalar et al. 2008) or phenotypic plasticity (Slepecky and Starmer 2009). Morphological changes of fungal strains on culture media after serial transfers have also been observed for other fungal species, for example Aspergillus flavus (Horn and Dorner 2002) and Metarhizium anisopliae (Ryan et al. 2002). The results in this study of the culturing of strain DTO 217-G5 showed, that phenotypic characteristics of various isolates of a single strain inoculated on the same media can differ widely. Although the original isolate DTO 217-G5 could accidently be a mixture of strains, the studied subcultures of DTO 217-G5 were likely to be pure single strains and they still showed phenotypic diversity. Regardless the phenotypic variation of these cultures, the ITS sequences were identical. This made the use of molecular techniques, instead of morphological characteristics, essential to identify the Aureobasidium isolates on species level.
Multi-locus DNA analysis
Several isolates had ambiguous nucleotide positions in their RPB2 sequence. The accidental presence of more than one strain in an examined isolate could explain these ambiguous nucleotide positions. In order to exclude the presence of multiple strains in a single isolate, biomass of an isolate was cultured in liquid media and after plating, separate colony forming units were used for PCR and sequencing; however, ambiguous nucleotide positions remained present in the sequences (unpublished data). Since RPB2 is regarded as a single-copy protein coding gene (Schmitt et al. 2009; Schoch et al. 2012), more tests are needed to study this phenomenon.
The reference data set for identification of Aureobasidium strains contained sixteen different described taxa (Table 3) and thirteen were shown in the Aureobasidium phylogenetic overview (Fig. 3). The distant relationship of K. harpospora (CBS 122914) to the Aureobasidium species and the placement of the K. microsticta strains (CBS 114.64 and CBS 342.66) in different phylogenetic clades is in concordance with Zalar et al. (2008) and Bills et al. (2012). The placement of Kabatiella zeae CBS 767.71 apart from the other Aureobasidium species can be supported by the outcome of a homology search of the ITS sequence of CBS 767.71 on GenBank: the best hit was Lecythophora sp. (KF624793.1).
Composition of Aureobasidium species
The composition of Aureobasidium species in a biofinish on wood can be studied with different techniques. However, no single technique is available that ensures the exact result (Nevalainen et al. 2015). It is well known that techniques that are based on culturing fungi have limitations that will influence the outcome of the fungal composition (Pitkäranta et al. 2008). For example: (a) conidia might produce more colonies on an agar plate than the same amount of biomass represented by hyphae (Pitt and Hocking 2009), (b) some fungi only grow on specific culturing conditions (Pitt and Hocking 2009; Samson et al. 2010), (c) some fungi are not culturable (Pitkäranta et al. 2011; Dei-Cas et al. 2006; Blackwell 2011) and (d) some species are overgrown by other fungi in mixed samples (Samson et al. 2010). Culture independent techniques, such as targeted cloning and sequencing of DNA regions or next generation amplicon sequencing, have been used increasingly over the last years to study the composition of fungal communities, for example in the area of soil ecology (Orgiazzi et al. 2013; Clemmensen et al. 2013), wood decay (Lindner et al. 2011; van der Wal et al. 2014) and human health (Ghannoum et al. 2010; Findley et al. 2013). Although culture independent techniques are the state of the art, they also have drawbacks. Each step of a DNA based method can introduce a bias. Examples are the differences in efficiency of DNA extraction per fungal species or morphologic structure (Saad et al. 2004; Fredricks et al. 2005) and the differences in efficiency of PCR amplification per species and primer set (Vainio and Hantula 2000; Bellemain et al. 2010). In the case of amplification of the ITS region, the species dependent number of copies of the targeted rDNA region per cell (Vilgalys and Gonzalez 1990; Simon and Weiss 2008; Lindner and Banik 2011) contributes to the different PCR amplification efficiencies. Another example of a bias is the absence of registration of taxa which are relatively scarcely present in a DNA mixture (Adams et al. 2013a; Prakitchaiwattana et al. 2004).
The origin of the fungal isolates, retrieved from the outdoor exposed specimens, is considered to be the concerned exposure site. The natural occurrence of Aureobasidium on outdoor exposed materials is well known and the distribution of Aureobasidium occurs by wind disturbance (Taylor et al. 2006), water drops (Hudson 1992; Madelin 1995) or insects (Zacchi and Vaughan-martini 2003; Pagnocca et al. 2008). Not only wood has been reported as outdoor substrate (Dix and Webster 1995; Schmidt 2006), but also other organic materials, such as leaves (Andrews et al. 2002; Woody et al. 2007), grapes (Prakitchaiwattana et al. 2004), as well as painted surfaces (Shirakawa et al. 2002; Kelley et al. 2006), plastic (Reynolds 1950; Webb et al. 2000), glass (Gorbushina and Palinska 1999; Schabereiter-Gurtner et al. 2001) and stone (Urzì et al. 2001; Ruibal et al. 2005). It should be noted that several wood samples in this study were subjected to unsterile handling and packaging.
Although analysis of bigger data sets and the use of other methods might generate a more complete view on the species compositions, the results obtained in this study indicated the predominance of A. melanogenum within the Aureobasidium population of biofinishes using a culture-based method. No deviation was observed among the different substrates of the long term exposed wood samples. The predominance of A. melanogenum in biofinishes on pine sapwood treated with raw linseed has been confirmed using a DNA-based method without a cultivation step. In order to confirm the indication that A. melanogenum is predominant within the Aureobasidium population of biofinishes generated on other substrates or at other exposition sites, more studies are needed. Also the potential predominance of this species within the entire fungal population of biofinishes should be investigated further. Next to Aureobasidium other wood stain fungi, such as species of Cladosporium and Sydowia, are to be expected (Schmidt 2006; Viitanen and Ritschkoff 2011). The use of a next generation sequencing approach, next to the culturing and PCR cloning method as used in this study, will allow a detailed compositional analysis (van Nieuwenhuijzen et al., publication in progress).
The assets of A. melanogenum in biofinish formation
The finding of A. melanogenum as native of biofinishes on oil treated wood is a first step in understanding and controlling biofinish formation. More research is recommended on the growth mechanisms of biofinishes. The deposition (natural inoculation), attachment, survival and reproduction of fungal fragments on the oil treated wood surface are all development steps of biofinish growth outdoors.
The natural inoculation of the substrate will be influenced by the natural occurrence of certain species at a specific location. Although Aureobasidium has been detected in outdoor air with short-term air sampling (Larsen and Gravesen 1991; Beaumont et al. 1985; Spicer and Gangloff 2005; Pyrri and Kapsanaki-Gotsi 2007) and outdoor located sedimentation plates (Urzì et al. 2001; Adams et al. 2013b), the occurrence of some Aureobasidium species seem to depend on the specific outdoor location. Both A. melanogenum and A. pullulans are widely spread and might be globally present species. A. melanogenum isolates in this study originated from five widespread locations and this species has been isolated outdoors by others in South Africa (CBS 131917, isolated by Van der Walt), Japan, Thailand and Norway (Zalar et al. 2008). Strains of A. pullulans originated from the Netherlands, South Africa, Australia and Norway (this study) and at least five other countries (Zalar et al. 2008). The absence of this species in the isolates originating from Cameroon could be explained by the relative low number of isolates (Fig. 5). In contrast, the widespread occurrence of various other Aureobasidium species is less likely, because the relative high number of isolates from the Netherlands only consisted of A. melanogenum and A. pullulans.
The results in this study on short term exposed samples indicated that in the first weeks of outdoor exposure A. pullulans was more present than A. melanogenum on raw linseed oil treated wood as well as on glass. This did not disturb the predominance of A. melanogenum in a later stage of the biofinish formation. Especially since composition of Aureobasidium species in time seemed different on glass, the dominant influence of developments steps other than deposition seems likely.
Thus far no data has been found as to why A. melanogenum is predominant within the Aureobasidium population of biofinishes on oil treated wood. It is currently unknown whether A. melanogenum is better in attachment, survival and/or reproduction on outdoor wood surfaces than other Aureobasidium species.
With respect to attachment: the biosynthesis of pullulan, an extracellular polymeric substance (EPS) adhesive, is described for at least four Aureobasidium species (Gostinčar et al. 2014) and also the production of other EPS, such as β-glucan and acidic polysaccharides, by different Aureobasidium species is known (Leal-Serrano et al. 1980; Hamada and Tsujisaka 1983; Yurlova and de Hoog 1997; Lotrakul et al. 2013).
Obviously, the production of melanin by Aureobasidium seems to be involved in its survival (Rättö et al. 2001; Hernández 2012; Nosanchuk and Casadevall 2003; Ruan et al. 2004; Paolo et al. 2006; Kogej et al. 2007). However, it is currently unknown whether A. melanogenum has an overall higher melanin content in comparison to the other species as may be suggested by its name. Genetic evidence, based on the presence of the number of genes possibly related to melanin synthesis, in an A. melanogenum strain (CBS 110374) and other full genome-sequenced Aureobasidium strains (Gostinčar et al. 2014) does not indicate obvious differences between the Aureobasidium species. Not only the amount, but also the type of melanin, that is produced by each species and the impact of different melanin types on specific stressors (e.g. UV, oxidizing agents) needs to be unravelled to understand the role of melanin. This requires an extensive investigation since many complicating factors are involved such as the difference in pigmentation of various isolates of a single A. melanogenum strain (Fig. 2), the existence of many other colours besides black in pigments resulting from melanin (Langfelder et al. 2003; Pal et al. 2014), the impact of exposure conditions on the amount of (unspecified) melanin produced by a single strain (Hernández and Evans 2015a, b) and the inability of easy melanin quantification methods, such as spectrophotometric measurements, to determine the type of melanin (Pal et al. 2014).
Next to survival, organisms need to multiply in order to support dark mould staining. Substrates are considered to play a role in this. One of the factors influenced by substrates is the availability of nutrients for fungal growth (van Nieuwenhuijzen et al. 2015). For example, Horvath et al. (1976) presumed that nutrients for A. pullulans formation on wood substrates are derived from the wood. Schoeman and Dickinson (1997) also concluded that this species uses nutrients derived from wood, in particularly the products of lignocellulosic photo degradation at weathered wood surfaces. However one should keep in mind that these referred studies were performed before the recognition of A. pullulans and A. melanogenum as separate species. Next to the wood also additional materials such as oil in the case of biofinishes on oil treated wood (van Nieuwenhuijzen et al. 2013, 2015) or the attracted organic matter such as pollen (Hudson 1992) might be used for growth of Aureobasidium. Possibly the nutrients on oil treated wood are more favourable for A. melanogum than other Aureobasidium species. More research is needed to understand the impact of substrates on the biofinish population.
Conclusions
The culture based study showed the common presence of A. melanogenum in biofinishes that were naturally formed outdoors on oil treated wood. This fungus was also commonly found on wood samples with non-biofinish mould staining. On most of the outdoor exposed wood samples that contained stained surfaces, A. melanogenum was isolated, regardless the type of (oil) treatment or wood species. A. melanogenum was detected on samples of all five widespread exposure sites. Other Aureobasidium species were detected on the wood samples as well, including several potentially new species in the case of the non-biofinish samples. The results indicated that the diversity of culturable Aureobasidium species depends on the geographical location of the exposure site. Larger data sets for these and other locations will be required to allow more defined conclusions. ITS-specific PCR, cloning and sequencing of biofinish DNA confirmed the predominance of A. melanogenum within the Aureobasidium population of biofinishes generated in the Netherlands on pine sapwood samples treated with raw linseed oil. To allow a detailed composition analysis of the entire fungal population of biofinishes, the use of data obtained with culturing, PCR cloning and a next generation sequencing approach is suggested for future works.
References
Adams RI, Amend AS, Taylor JW, Bruns TD (2013a) A unique signal distorts the perception of species richness and composition in high-throughput sequencing surveys of microbial communities: a case study of fungi in indoor dust. Microb Ecol 66:735–741. doi:10.1007/s00248-013-0266-4
Adams RI, Miletto M, Taylor JW, Bruns TD (2013b) Dispersal in microbes: fungi in indoor air are dominated by outdoor air and show dispersal limitation at short distances. ISME J 7:1262–1273. doi:10.1038/ismej.2013.28
Andrews JH, Spear RN, Nordheim EV (2002) Population biology of Aureobasidium pullulans on apple leaf surfaces. Can J Microbiol 48(6):500–513. doi:10.1139/W02-044
Bardage SL (1998) Susceptibility of painted wood to Aureobasidium pullulans: fungal stain and growth patterns. Holz als Roh- und Werkstoff 56:359–364
Beaumont F, Kauffman HF, van der Mark TH, Sluiter HJ, de Vries K (1985) Volumetric aerobiological survey of conidial fungi in the North-East Netherlands. I. Seasonal patterns and the influence of metereological variables. Allergy 40:173–180
Bellemain E, Carlsen T, Brochmann C, Coissac E, Taberlet P et al (2010) ITS as an environmental DNA barcode for fungi: an in silico approach reveals potential PCR biases. BMC Microbiol 10:189. doi:10.1186/1471-2180-10-189
Bills GF, Menéndez VG, Platas G (2012) Kabatiella bupleuri sp. nov. (Dothideales), a pleomorphic epiphyte and endophyte of the Mediterranean plant Bupleurum gibraltarium (Apiaceae). Mycologia 104:962–973. doi:10.3852/12-003
Blackwell M (2011) The fungi: 1, 2, 3… 5.1 million species? Am J Bot 98:426–438. doi:10.3732/ajb.1000298
Clemmensen KE, Bahr A, Ovaskainen O, Dahlberg A, Ekblad A et al (2013) Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science 339:1615–1618. doi:10.1126/science.1231923
Crous PW, Summerell BA, Swart L, Denman S, Taylor JE, Bezuidenhout CM, Palm ME, Marincowitz S, Groenewald JZ (2011) Fungal pathogens of Proteaceae. Persoonia 27:20–45. doi:10.3767/003158511X606239
de Hoog GS, Gerrits van den Ende AH (1998) Molecular diagnostics of clinical strains of filamentous Basidiomycetes. Mycoses 41:183–189. doi:10.1111/j.1439-0507.1998.tb00321.x
Dei-Cas E, Chabé M, Moukhlis R, Durand-Joly I, Aliouat el M, Stringer JR, Cushion M, Noël C, de Hoog GS, Guillot J, Viscogliosi E (2006) Pneumocystis oryctolagi sp. nov., an uncultured fungus causing pneumonia in rabbits at weaning: review of current knowledge, and description of a new taxon on genotypic, phylogenetic and phenotypic bases. FEMS Microbiol Rev 30:853–871
Dickinson DJ (1972) Disfigurement of decorative timbers by blue stain fungi. Int Pest Control 3:21–25
Difco Laboratories (1998) Difco manual, 11th edn. Becton Dickinson and Company, Maryland
Dix NJ, Webster J (1995) Fungal ecology. Chapman and Hall, London
EN113 (1996) Wood preservatives—test method for determining the protective effectiveness against wood destroying basidiomycetes—determination of the toxic values. European Committee for Standardization
Filippovych K, Huinink H, Ven van der L, Adan OCG (2015) Characterization of biofilm formation on wood treated with vegetable oils by color-based image interpretation method. In: Proceedings of the International Research Group on Wood Preservation, Vina del Mar, IRG/WP 15-40697
Findley K, Oh J, Yang J, Conlan S, Deming C, Meyer JA, Schoenfeld D, Nomicos E, Park M, NIH Intramural Sequencing Center Comparative Sequencing Program, Kong HH, Segre JA (2013) Topographic diversity of fungal and bacterial communities in human skin. Nature 498:367–370. doi:10.1038/nature12171
Fredricks DN, Smith C, Meier A (2005) Comparison of six DNA extraction methods for recovery of fungal DNA as assessed by quantitative PCR. J Clin Microbiol 43:5122–5128. doi:10.1128/JCM.43.10.5122-5128.2005
Fritsche W, Laplace F (1999) Mikrobiologie, 2 Aufl. edn. Spektrum Akademischer Verlag, Heidelberg
Gaur R, Singh R, Gupta M, Gaur MK (2010) Aureobasidium pullulans, an economically important polymorphic yeast with special reference to pullulan. Afr J Biotechnol 9:7989–7997. doi:10.5897/AJB10.948
Ghannoum MA, Jurevic RJ, Mukherjee PK, Cui F, Sikaroodi M, Naqvi A, Gillevet PM (2010) Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS pathog 6:e1000713. doi:10.1371/journal.ppat.1000713
Gobakken LR, Westin M (2008) Surface mould growth on five modified wood substrates coated with three different coating systems when exposed outdoors. Int Biodeterior Biodegr 62:397–402. doi:10.1016/j.ibiod.2008.03.004
Gorbushina AA, Palinska KA (1999) Biodeteriorative processes on glass: experimental proof of the role of fungi and cyanobacteria. Aerobiologia 15:183–192. doi:10.1023/A:1007616614172
Gostinčar C, Ohm RA, Kogej T, Sonjak S, Turk M, Zajc J, Zalar P, Grube M, Sun H, Han J, Sharma A, Chiniquy J, Ngan CY, Lipzen A, Barry K, Grigoriev IV, Gunde-Cimerman N (2014) Genome sequencing of four Aureobasidium pullulans varieties: biotechnological potential, stress tolerance, and description of new species. BMC Genom 15:549. doi:10.1186/1471-2164-15-549
Hamada N, Tsujisaka Y (1983) The structure of the carbohydrate moiety of an acidic polysaccharide produced by Aureobasidium sp. K-1. Agric Biol Chem 47:1167–1172. doi:10.1080/00021369.1983.10866085
Hernández VA (2012) Role of non-decay fungi on the weathering of wood. Electronic Theses and Dissertations (ETDs) 2008+. University of British Colombia, Vancouver
Hernández VA, Evans PD (2015a) Effects of UV radiation on melanization and growth of fungi isolated from weathered wood surfaces. IRG/WP 15-10842. The International Research Group on Wood Protection, Stockholm
Hernández VA, Evans PD (2015b) Technical note: melanization of the wood-staining fungus Aureobasidium pullulans in response to UV radiation. Wood Fiber Sci 47:120–124
Horn BW, Dorner JW (2002) Effect of competition and adverse culture conditions on aflatoxin production by Aspergillus flavus through successive generations. Mycologia 94:741–751
Horvath RS, Brent MM, Cropper DG (1976) Paint deterioration as a result of the growth of Aureobasidium pullulans on wood. Appl Environ Microbiol 32:505–507
Houbraken J (2013) Revealing relationships in Trichocomaceae, with the emphasis on Penicillium, Dissertation, University of Utrecht
Hudson HJ (1992) Fungal biology. Press Syndicate of the University of Cambridge, Cambridge
Kelley J, Kennedy R, Kinsey G, Springle WR (2006) Statistical methods applied to field colonisation of coatings by fungi, part: 3, ecology analysis. Surface Coatings Int, Part A, Coating J 89:91–95
Kogej T, Stein M, Volkmann M, Gorbushina AA, Galinski EA, Gunde-Cimerman N (2007) Osmotic adaptation of the halophilic fungus Hortaea werneckii: role of osmolytes and melanization. Microbiology 153:4261–4273. doi:10.1099/mic.0.2007/010751-0
Langfelder K, Streibel M, Jahn B, Haase G, Brakhage AA (2003) Biosynthesis of fungal melanins and their importance for human pathogenic fungi. Fungal Gen Biol 38:143–158. doi:10.1016/S1087-1845(02)00526-1
Larsen L, Gravesen S (1991) Seasonal variation of outdoor airborne viable microfungi in Copenhagen, Denmark. Grana 30:467–471. doi:10.1080/00173139109432011
Leal-Serrano G, Ruperez P, Leal JA (1980) Acidic polysaccharide from Aureobasidium pullulans. Trans Br Mycol Soc 75:57–62. doi:10.1016/S0007-1536(80)80194-X
Lindner DL, Banik MT (2011) Intragenomic variation in the ITS rDNA region obscures phylogenetic relationships and inflates estimates of operational taxonomic units in genus Laetiporus. Mycologia 103:731–740. doi:10.3852/10-331
Lindner DL, Vasaitis R, Kubartová A, Allmer J, Johannesson H, Banik MT, Stenlid J (2011) Initial fungal colonizer affects mass loss and fungal community development in Picea abies logs 6 yr after inoculation. Fungal Ecol 4:449–460. doi:10.1016/j.funeco.2011.07.001
Lotrakul P, Unhapattaratitikul P, Seelanan T, Prasongsuk S, Punnapayak H (2013) An aubasidan-like β-glucan produced by Aureobasidium pullulans in Thailand. SCIENCEASIA 39:363–368. doi:10.2306/scienceasia1513-1874.2013.39.363
Madelin TM, Madelin MF (1995) Biological analysis of fungi and associated molds. In: Cox CS, Wathes CM (eds) Bioaerosols handbook. Lewis Publishers, Florida, pp 361–386
Manitchotpisit P, Leathers TD, Peterson SW, Kurtzman CP, Li X-L, Eveleigh DE, Lotrakul P, Prasongsuk S, Dunlap CA, Vermillion KE, Punnapayak H (2009) Multilocus phylogenetic analyses, pullulan production and xylanase activity of tropical isolates of Aureobasidium pullulans. Mycol Res 113:1107–1120. doi:10.1016/j.mycres.2009.07.008
Masclaux F, Guého H, de Hoog GS, Christen R (1995) Phylogenetic relationships of human-pathogenic Cladosporium (Xylohypha) species inferred from partial LSU rRNA sequences. J Med Vet Mycol 33:327–338. doi:10.1080/02681219580000651
Nevalainen A, Täubel M, Hyvärinen A (2015) Indoor fungi: companions and contaminants. Indoor Air 25:125–156. doi:10.1111/ina.12182
Nosanchuk JD, Casadevall A (2003) The contribution of melanin to microbial pathogenesis. Cell Microbiol 5:203–223. doi:10.1046/j.1462-5814.2003.00268.x
Orgiazzi A, Bianciotto V, Bonfante P, Daghino S, Ghignone S, Lazzari A et al (2013) 454 pyrosequencing analysis of fungal assemblages from geographically distant, disparate soils reveals spatial patterning and a core mycobiome. Diversity 5:73–98. doi:10.3390/d5010073
Pagnocca FC, Rodrigues A, Nagamoto NS, Bacci M Jr (2008) Yeasts and filamentous fungi carried by the gynes of leaf-cutting ants. Antonie Van Leeuwenhoek 94:517–526. doi:10.1007/s10482-008-9268-5
Pal AK, Gajjar DU, Vasavada AR (2014) DOPA and DHN pathway orchestrate melanin synthesis in Aspergillus species. Med Mycol 52:10–18. doi:10.3109/13693786.2013.826879
Paolo WF Jr, Dadachova E, Mandal P, Casadevall A, Szaniszlo PJ, Nosanchuk JD (2006) Effects of disrupting the polyketide synthase gene WdPKS1 in Wangiella [Exophiala] dermatitidis on melanin production and resistance to killing by antifungal compounds, enzymatic degradation, and extremes in temperature. BMC Microbiol 6:55. doi:10.1186/1471-2180-6-55
Peterson SW, Manitchotpisit P, Leathers TD (2013) Aureobasidium thailandense sp. nov. isolated from leaves and wooden surfaces. Int J Syst Evol Microbiol 63:790–795. doi:10.1099/ijs.0.047613-0
Pitkäranta M, Meklin T, Hyvärinen A, Paulin L, Auvinen P, Nevalainen A, Rintala H (2008) Analysis of fungal flora in indoor dust by ribosomal DNA sequence analysis, quantitative PCR, and culture. Appl Environ Microbiol 74:233–244. doi:10.1128/AEM.00692-07
Pitkäranta M, Meklin T, Hyvärinen A, Nevalainen A, Paulin L, Auvinen P, Lignell U, Rintala H (2011) Molecular profiling of fungal communities in moisture damaged buildings before and after remediation–a comparison of culture-dependent and culture-independent methods. BMC Microbiol 11:235. doi:10.1186/1471-2180-11-235
Pitt JI, Hocking AD (2009) Fungi and food spoilage, 3rd edn. Springer, Dordrecht
Prakitchaiwattana CJ, Fleet GH, Heard GM (2004) Application and evaluation of denaturing gradient gel electrophoresis to analyse the yeast ecology of wine grapes. FEMS Yeast Res 4:865–877. doi:10.1016/j.femsyr.2004.05.004
Pyrri I, Kapsanaki-Gotsi E (2007) A comparative study on the airborne fungi in Athens, Greece, by viable and non-viable sampling methods. Aerobiologia 23:3–15. doi:10.1007/s10453-006-9039-6
Rättö M, Chatani M, Ritschkoff AC, Viikari L (2001) Screening of micro-organisms for decolorization of melanins produced by bluestain fungi. Appl Microbiol Biotechnol 55:210–213. doi:10.1007/s002530000516
Reynolds ES (1950) Pullularia as a cause of deterioration of paint and plastic surfaces in South Florida. Mycologia 42(3):432–448
Ruan L, Yu Z, Fang B, He W, Wang Y, Shen P (2004) Melanin pigment formation and increased UV resistance in Bacillus thuringiensis following high temperature induction. Syst Appl Microbiol 27:286–289. doi:10.1078/0723-2020-00265
Ruibal C, Platas G, Bills GF (2005) Isolation and characterization of melanized fungi from limestone formations in Mallorca. Mycol Progr 4:23–38
Ryan MJ, Bridge PD, Smith D, Jeffries P (2002) Phenotypic degeneration occurs during sector formation in Metarhizium anisopliae. J Appl Microbiol 93:163–168. doi:10.1046/j.1365-2672.2002.01682.x
Saad DS, Kinsey GC, Kim S, Gaylarde CC (2004) Extraction of genomic DNA from filamentous fungi in biofilms on water-based paint coatings. Int Biodegr Biodeterior 54:99–103. doi:10.1016/j.ibiod.2004.05.003
Sailer MF, van Nieuwenhuijzen EJ, Knol W (2010) Forming of a functional biofilm on wood surfaces. Ecol Eng 36:163–167. doi:10.1016/j.ecoleng.2009.02.004
Samson RA, Hoekstra ES, Frisvad JC (2004) Introduction to food-borne fungi, 7th edn. CBS-KNAW Fungal Biodiversity Centre, Utrecht
Samson RA, Houbraken J, Frisvad JC, Thrane U, Andersen B (2010) Food and indoor fungi. CBS Laboratory Manual series 2. CBS-KNAW Fungal Biodiversity Centre, Utrecht
Schabereiter-Gurtner C, Piñar G, Lubitz W, Rölleke S (2001) Analysis of fungal communities on historical church window glass by denaturing gradient gel electrophoresis and phylogenetic 18S rDNA sequence analysis. J Microbiol Methods 47:345–354. doi:10.1016/S0167-7012(01)00344-X
Schmidt O (2006) In: Czeschlik D (ed) Wood and tree fungi: biology, damage, protection, and use. Springer, Berlin
Schmitt I, Crespo A, Divakar PK, Fankhauser JD, Herman-Sackett E, Kalb K, Nelsen MP, Nelson NA, Rivas-Plata E, Shimp AD, Widhelm T, Lumbsch HT (2009) New primers for promising single-copy genes in fungal phylogenetics and systematics. Persoonia 23:35–40. doi:10.3767/003158509X470602
Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA et al (2012) Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc Natl Acad Sci USA 109:6241–6246. doi:10.1073/pnas.1117018109
Schoeman M, Dickinson D (1997) Growth of Aureobasidium pullulans on lignin breakdown products at weathered wood surfaces. Mycologist 11:168–172. doi:10.1016/S0269-915X(97)80095-X
Shirakawa MA, Gaylarde CC, Gaylarde PM, John V, Gambale W (2002) Fungal colonization and succession on newly painted buildings and the effect of biocide. FEMS Microbiol Ecol 39:165–173. doi:10.1111/j.1574-6941.2002.tb00918.x
Simon UK, Weiss M (2008) Intragenomic variation of fungal ribosomal genes is higher than previously thought. Mol Biol Evol 25:2251–2254. doi:10.1093/molbev/msn188
Slepecky RA, Starmer WT (2009) Phenotypic plasticity in fungi: a review with observations on Aureobasidium pullulans. Mycologia 101:823–832. doi:10.3852/08-197
Spicer R, Gangloff H (2005) Establishing site specific reference levels for fungi in outdoor air for building evaluation. J Occup Environ Hyg 2:257–266. doi:10.1080/15459620590946401
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi:10.1093/molbev/msr121
Taylor JW, Jacobson DJ, Kroken S, Kasuga T, Geiser DM, Hibbett DS, Fisher MC (2000) Phylogenetic species recognition and species concepts in fungi. Fungal Genet Biol 31:21–32. doi:10.1006/fgbi.2000.1228
Taylor PE, Esch R, Flagan RC, House J, Tran L, Glovsky MM (2006) Identification and possible disease mechanisms of an under-recognized fungus, Aureobasidium pullulans. Int Arch Allergy Immunol 139:45–52. doi:10.1159/000089522
Thambugala KM, Ariyawansa HA, Li YM, Boonmee S, Hongsanan S, Tian Q et al (2014) Dothideales. Fungal Divers 68:105–158. doi:10.1007/s13225-014-0303-8
Urzì C, De Leo F, Salamone P, Criseo G (2001) Airborne fungal spores colonising marbles exposed in the terrace of Messina Museum, Sicily. Aerobiologia 17:11–17. doi:10.1023/A:1007652300354
Vainio EJ, Hantula J (2000) Direct analysis of wood-inhabiting fungi using denaturing gradient gel electrophoresis of amplified ribosomal DNA. Mycol Res 104:927–936
van der Wal A, Ottosson E, de Boer W (2014) Neglected role of fungal community composition in explaining variation in wood decay rates. Ecol 96:124–133. doi:10.1890/14-0242.1
Van Nieuwenhuijzen EJ, Sailer MF, Samson RA, Adan OCG (2013) Detection of Aureobasidium as the dominant fungus on coated wood. In: Proceedings of the International Research Group on Wood Preservation, Stockholm, IRG/WP 13-10796
van Nieuwenhuijzen EJ, Sailer MF, Gobakkend LR, Adan OCG, Punt PJ, Samson RA (2015) Detection of outdoor mould staining as biofinish on oil treated wood. Int Biodegr Biodeterior 105:215–227
Viitanen H, Ritschkoff A (2011) Coating and surface treatment of wood. In: Adan OCG, Samson RA (eds) Fundamentals of mold growth in indoor environments and strategies for healthy living. Wageningen Academic Publishers, Wageningen, pp 463–488
Vilgalys D, Gonzalez D (1990) Organization of ribosomal DNA in the basidiomycete Thanatephorus praticola. Curr Genet 18:277–280. doi:10.1007/BF00318394
Webb JS, Nixon M, Eastwood IM, Greenhalgh M, Robson GD, Handley PS (200) Fungal colonization and biodeterioration of plasticized polyvinyl chloride. Appl Environ Microbiol 66:3194–200. doi: 10.1128/AEM.66.8.3194-3200.2000
Wijayawardene NN, Crous PW, Kirk PM, Hawksworth DL, Boonmee S, Braun U et al (2014) Naming and outline of Dothideomycetes–2014 including proposals for the protection or suppression of generic names. Fungal Divers 69:1–55. doi:10.1007/s13225-014-0309-2
Woody ST, Ives AR, Nordheim EV, Andrews JH (2007) Dispersal, density dependence, and population dynamics of a fungal microbe on leaf surfaces. Ecology 88:1513–1524
Yilmaz N, Visagie CM, Houbraken J, Frisvad JC, Samson RA (2014) Polyphasic taxonomy of the genus Talaromyces. Stud Mycol 78:175–341. doi:10.1016/j.simyco.2014.08.001
Yurlova NA, de Hoog GS (1997) A new variety of Aureobasidium pullulans characterised by exopolysaccharide structure, nutritional physiology and molecular features. Antonie Van Leeuwenhoek 72:141–147. doi:10.1023/A:1000212003810
Yurlova NA, de Hoog GS, Van den Ende AHGG (1999) Taxonomy of Aureobasidium and allied genera. Stud Mycol 43:63–69
Zacchi L, Vaughan-Martini A (2003) Distribution of three yeast and yeast-like species within a population of soft scale insects (Saissetia oleae) as a function of developmental age. Ann Microbiol 53:43–46
Zalar P, Gostincar C, de Hoog GS, Ursic V, Sudhadham M, Gunde-Cimerman N (2008) Redefinition of Aureobasidium pullulans and its varieties. Stud Mycol 61:21–38. doi:10.3114/sim.2008.61.02
Acknowledgments
The authors acknowledge Karl Rumbold (University of the Witwatersrand), Herbert Reef (Houthandel RTT) Manon Timmermans (Life Without Barriers) and Lone Ross Gobakken (NIBIO, Norwegian Institute of Bioeconomy Research) for the outdoor exposure of wood samples. The authors thank Peter Punt (Dutch DNA Biotech) for his comments, Stephanie Rensink for her technical assistance and Frank Segers (CBS- KNAW Fungal Biodiversity Centre) for his lectures on melanin. This research is supported by the Dutch Technology Foundation STW, which is part of the Netherlands Organisation for Scientific Research (NWO), and which is partly funded by the Ministry of Economic Affairs. Partners of this STW research project are CBS-KNAW Fungal Biodiversity Centre, Eindhoven University of Technology, TNO, Lambert van den Bosch, Stiho, Touchwood and Regge Hout.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
van Nieuwenhuijzen, E.J., Houbraken, J.A.M.P., Meijer, M. et al. Aureobasidium melanogenum: a native of dark biofinishes on oil treated wood. Antonie van Leeuwenhoek 109, 661–683 (2016). https://doi.org/10.1007/s10482-016-0668-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-016-0668-7