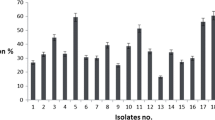
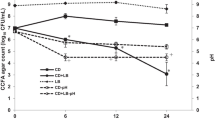
Abstract
Toxigenic strains of Clostridium difficile were co-cultured with different strains of bifidobacteria and lactobacilli. Spent culture supernatants were tested for biological activity on cultured Vero cells. Co-culture of C. difficile with some potentially probiotic strains lead to a reduction of the biological activity of spent culture supernatants. The observed effects cannot be ascribed either to secreted factors from the probiotic strains or to toxin adsorption by bacterial cells. Immunological assays showed that there was significant diminution of both clostridial toxins (TcdA and TcdB) in spent culture supernatants of co-cultures as compared with pure clostridial cultures. Even though co-cultured clostridial cells showed a slight increase of intracellular toxins, this increase did not completely explains the reduction of toxin concentration in culture supernatants. The evidence suggests that the antagonism could be due to the diminution of the synthesis and/or secretion of both clostridial toxins. Our findings provide new insights into the possible mechanisms involved in the protective effect of probiotics in the context of C. difficile infection.






Similar content being viewed by others
Abbreviations
- SCS:
-
Spent culture supernatants
- TcdA:
-
C. difficile toxin A
- TcdB:
-
C. difficile toxin B
- CDAD:
-
C. difficile associated diarrhea
- OD600 nm :
-
Optical density at 600 nm
- PBS:
-
Phosphate buffered saline
- DMEM:
-
Dulbbeco’s Modified Eagle’s Medium
- rd:
-
Ratio of detached cells
- NBT:
-
Nitro-blue tetrazolium chloride
- BCIP:
-
5-Bromo-4-chloro-3′-indolyphosphate p-toluidine salt
- DD50:
-
Dose of SCS that leads to the detachment of 50% of the cells
References
Banerjee P, Merkel GJ, Bhunia AK (2009) Lactobacillus delbrueckii ssp. bulgaricus B-30892 can inhibit cytotoxic effects and adhesion of pathogenic Clostridium difficile to Caco-2 cells. Gut Pathog 1:8–19
Bélanger SD, Boissinot M, Clairoux N, Picard FJ, Bergeron MG (2003) Rapid detection of Clostridium difficile in feces by Real-time PCR. J Clin Microbiol 41:730–734
Benyacoub J, Pérez PF, Rochat F, Saudan KY, Reuteler G, Antille LN, Humen MA, De Antoni GL, Cavadini C, Blum S, Schiffrin EJ (2005) Enterococcus faecium strain SF68 improves immune response to Giardia intestinalis in mice. J Nutr 135:1171–1176
Castagliuolo I, Keates AC, Wang CC, Pasha A, Valenick L, Kelly CP, Nikulasson ST, LaMont JT, Pothoulakis C (1998) Clostridium difficile toxin A stimulates macrophage-inflammatory protein-2 production in rat intestinal epithelial cells. J Immunol 160:6039–6045
Chen X, Kokkotou EG, Mustafa N, Bhaskar KR, Sougioultzis S, Pothoulakis C, Kelly CP (2006) Saccharomyces boulardii inhibits ERK1/2 mitogen activated protein kinase activation both in vitro and in vivo and protects against Clostridium difficile toxin A-induced enteritis. J Biol Chem 281:24449–24454
Collado MC, Gueimonde M, Hernández M, Sanz Y, Salminen S (2005) Adhesion of selected Bifidobacterium strains to human intestinal mucus and the role of adhesion in enteropathogen exclusion. J Food Prot 68:2672–2678
Colombel JF, Cortot A, Neut C, Romond C (1987) Yoghurt with Bifidobacterium longum reduces erythromycin-induced gastrointestinal effects. Lancet 2:43
Dineen SS, Villapakkam AC, Nordman JT, Sonenshein AL (2007) Repression of Clostridium difficile gene expression by CodY. Mol Microbiol 66:206–219
Dubberke ER, Reske KA, Yan Y, Olsen MA, McDonald LC, Fraser VJ (2007) Clostridium difficile-associated disease in a setting of endemicity: identification of novel risk factors. Clin Infect Dis 45:1543–1549
Dupuy B, Sonenshein AL (1998) Regulated transcription of Clostridium difficile toxin genes. Mol Microbiol 27:107–120
Garrote GL, Abraham AG, De Antoni AG (2001) Chemical and microbiological characterisation of kefir grains. J Dairy Res 68:639–652
Gomez Zavaglia A, Kociubinski G, Pérez PF, De Antoni GL (1998) Isolation and characterization of Bifidobacterium strains for probiotic formulation. J Food Prot 61:865–873
Govind R, Vediyappan G, Rolfe RD, Fralik JA (2006) Evidence that Clostridium difficile TcdC is a membrane-associated protein. J Bacteriol 188:3716–3720
Gursoy S, Guven K, Arikan T, Yurci A, Torun E, Baskol M, Ozbakir O, Yucesoy M (2007) Clostridium difficile infection frequency in patients with nosocomial infections or using antibiotics. Hepatogastroenterology 54:1720–1724
Hugo AA, Kakisu EJ, De Antoni GL, Pérez PF (2008) Lactobacilli antagonize biological effects of enterohaemorrhagic Escherichia coli in vitro. Lett Appl Microbiol 46:613–619
Humen MA, De Antoni GL, Benyacoub J, Costas ME, Cardozo MI, Kozubsky L, Saudan K-Y, Boenzli-Bruand A, Blum S, Schiffrin EJ, Pérez PF (2005) Lactobacillus johnsonii La1 antagonizes Giardia intestinalis in vivo. Infect Immun 73:1265–1269
Hundsberger T, Braun V, Weidmann M, Leukel P, Sauerborn M, Von Eichel-Streiber C (1997) Transcription analysis or the genes tcdA-E of the pathogenicity locus of Clostridium difficile. Eur J Biochem 244:735–742
Jank T, Giesemann T, Aktories K (2007) Rho-glucosylating Clostridium difficile toxins A and B: new insights into structure and function. Glycobiology 17:15R–22R
Karlsson S, Lindberg A, Norin E, Burman LG, Akerlund T (2000) Toxins, butyric acid, and short-chain fatty acids are coordinately expressed and down-regulated by cysteine in Clostridium difficile. Infect Immun 68:5881–5888
Karlsson S, Burman LG, Akerlund T (2008) Induction of toxins in Clostridium difficile is associated with dramatic changes of its metabolism. Microbiology 154:3430–3436
Kotowska M, Albrecht P, Szakewska H (2005) Saccharomyces boulardii in the prevention of antibiotic-associated diarrhea in children: a randomized double-blind placebo-controlled trial. Aliment Pharmacol Ther 21:583–590
Lewis S, Burmeister S, Brazier J (2005) Effect of the probiotic oligofructose on relapse of Clostridium difficile-associated diarrhea: a randomized, controlled study. Clin Gastroenterol Hepatol 3:442–448
Limaye AP, Turgeon DK, Cookson BT, Fritsche TR (2000) Pseudomembranous colitis caused by a toxin A(−) B(+) strain of Clostridium difficile. J Clin Microbiol 38:696–697
Lyerly DM, Krivan HC, Wilkins TD (1988) Clostridium difficile: its disease and toxins. Clin Microbiol Rev 1:1–18
Maegawa T, Karasawa T, Ohta T, Wang X, Kato H, Hayashi H, Nakamura S (2002) Linkage between toxin production and purine biosynthesis in Clostridium difficile. J Med Microbiol 51:34–41
Mani N, Dupuy B (2001) Regulation of toxin synthesis in Clostridium difficile by an alternative RNA polymerase sigma factor. PNAS 98:5844–5849
Matsuki S, Ozaki E, Shozu M, Inoue M, Shimizu S, Yamaguchi N, Karasawa T, Yamagishi T, Nakamura S (2005) Colonization by Clostridium difficile of neonates in a hospital, and infants and children in three day-care facilities of Kanazawa, Japan. Int Microbiol 8:43–48
Medrano M, Pérez PF, Abraham AG (2008) Kefiran antagonizes cytopathic effects of Bacillus cereus extracellular factors. Int J Food Microbiol 122:1–7
Medrano M, Hamet MF, Abraham AG, Pérez PF (2009) Kefiran protects Caco-2 cells from cytopathic effects induced by Bacillus cereus infection. Antonie van Leewenhoek 96:505–513
Minnaard J, Humen M, Pérez PF (2001) Effect of Bacillus cereus exocellular factors on human intestinal epithelial cells. J Food Prot 64:1535–1541
Minnaard J, Lievin-Le Moal V, Coconnier MH, Servin AL, Pérez PF (2004) Disassembly of F-actin cytoskeleton after interaction of Bacillus cereus with fully differentiated human intestinal Caco-2 cells. Infect Immun 72:3106–3112
Nakamura S, Mikawa M, Tanabe N, Yamakawa K, Nishida S (1982) Effect of clindamycin on cytotoxin production by Clostridium difficile. Microbiol Immunol 26:985–992
Niers LE, Hoekstra MO, Timmerman HM, van Uden NO, de Graaf PM, Smits HH, Kimpen JL, Rijkers GT (2007) Selection of probiotic bacteria for prevention of allergic diseases: immunomodulation of neonatal dendritic cells. Clin Exp Immunol 149:344–352
Osgood DP, Wood NP, Sperry JF (1993) Nutritional aspects of cytotoxin production by Clostridium difficile. Appl Environ Microbiol 59:3985–3988
Perez PF, Minnaard J, Disalvo EA, De Antoni GL (1998) Surface properties of bifidobacterial strains of human origin. Appl Environ Microbiol 64:21–26
Plummer S, Weaver MA, Harris JC, Dee P, Hunter J (2004) Clostridium difficile pilot study: effects of probiotic supplementation on the incidence of C. difficile diarrhoea. Int Microbiol 7:59–62
Rönnqvist D, Forsgren-Brusk U, Husmark U, Grahn-Håkansson E (2007) Lactobacillus fermentum Ess-1 with unique growth inhibition of vulvo-vaginal candidiasis pathogens. J Med Microbiol 56:1500–1504
Ruas-Madiedo P, Gueimonde M, Margolles A, de los Reyes-Gavilán CG, Salminen S (2006) Exopolysaccharides produced by probiotic strains modify the adhesion of probiotics and enteropathogens to human intestinal mucus. J Food Prot 69:2011–2015
Schirmer J, Aktories K (2004) Large clostridial cytotoxins: cellular biology of Rho/Ras-glucosylating toxins. Biochim Biophys Acta 1673:66–74
Schroeder M (2005) Clostridium difficile associated diarrhea. Am Fam Physician 71:921–928
Segarra-Newnham M (2007) Probiotics for Clostridium difficile-associated diarrhea: focus on Lactobacillus rhamnosus GG and Saccharomyces boulardii. Ann Pharmacother 41:1284–1287
Sunenshine RH, McDonald LC (2006) Clostridium difficile-associated disease: new challenges from an established pathogen. Cleve Clin J Med 73:187–197
Trejo FM, Minnaard J, Pérez PF, De Antoni GL (2006) Inhibition of Clostridium difficile growth and adhesion to enterocytes by Bifidobacterium supernatants. Anaerobe 12:186–193
Wullt M, Hagslatt ML, Odenholt I (2003) Lactobacillus plantarum 299v for the treatment of recurrent Clostridium difficile-associated diarrhoea: a double-blind, placebo-controlled trial. Scand J Infect Dis 35:365–367
Yamakawa K, Karasawa T, Ohta T, Hayashi H, Nakamura S (1998) Inhibition of enhanced toxin production by Clostridium difficile in biotin-limited conditions. J Med Microbiol 47:767–771
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Trejo, F.M., Pérez, P.F. & De Antoni, G.L. Co-culture with potentially probiotic microorganisms antagonises virulence factors of Clostridium difficile in vitro. Antonie van Leeuwenhoek 98, 19–29 (2010). https://doi.org/10.1007/s10482-010-9424-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-010-9424-6